Liposome combined medicine and method for preparing the same
A liposome and drug technology, applied in liposome delivery, pharmaceutical formulations, antibacterial drugs, etc., can solve the problems of blood white blood cell and thrombocytopenia, abnormal liver and kidney function, easy to cause phlebitis, etc., and achieve the goal of drug The effect of reducing the amount of distribution, reducing the number of medications, and enhancing the curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The formula that present embodiment adopts is as follows:
[0046] Mixture of soybean lecithin and dipalmitoylcholine (3:1) 0.05mol
[0047] Cholesterol and β-sitosterol mixture (1:1) 0.05mol
[0048] Clindamycin Hydrochloride and Levofloxacin Lactate Random Ratio Mixture 200mg
[0049] Reduced glutathione and tiopronin mixture (molar ratio 1:3) 20g
[0050]Polyethylene glycol 6000 0.005mol
[0051] Sodium glutamate 30g
[0052] The number of grams contained in the mixture of Pluronic F68 and hypromellose in any ratio in 100ml liquid medicine is 0.2%-0.7%g / ml
[0053] Phosphate buffer 0.015M pH3.0-5.5 added to 1000ml
[0054] The 200 mg of the arbitrary ratio mixture of clindamycin hydrochloride and levofloxacin lactate is calculated based on the amount of clindamycin and levofloxacin.
[0055] Preparation steps:
[0056] (1) Dissolve the mixture of soybean lecithin and dipalmitoylcholine, the mixture of cholesterol and β-sitosterol in an appropriate amount of ch...
Embodiment 2
[0067] The formula that present embodiment adopts is as follows:
[0068] Mixture of soybean lecithin and dipalmitoylcholine (5:1) 0.1mol
[0069] Cholesterol and β-sitosterol mixture (2:1) 0.1mol
[0070] Clindamycin Hydrochloride and Levofloxacin Hydrochloride Random Ratio Mixture 400mg
[0071] Reduced glutathione and tiopronin mixture (molar ratio 1:5) 30g
[0072] Polyethylene glycol 6000 0.001mol
[0073] Sodium glutamate 40g
[0074] The number of grams contained in the mixture of Pluronic F68 and hypromellose in any ratio in 100ml liquid medicine is 0.2%-0.7%g / ml
[0075] Phosphate buffer 0.015M pH3.0-5.5 added to 1000ml
[0076] The 200 mg of the arbitrary ratio mixture of clindamycin hydrochloride and levofloxacin hydrochloride is calculated based on the amount of clindamycin hydrochloride and levofloxacin hydrochloride.
[0077] Preparation steps: with reference to Example 1.
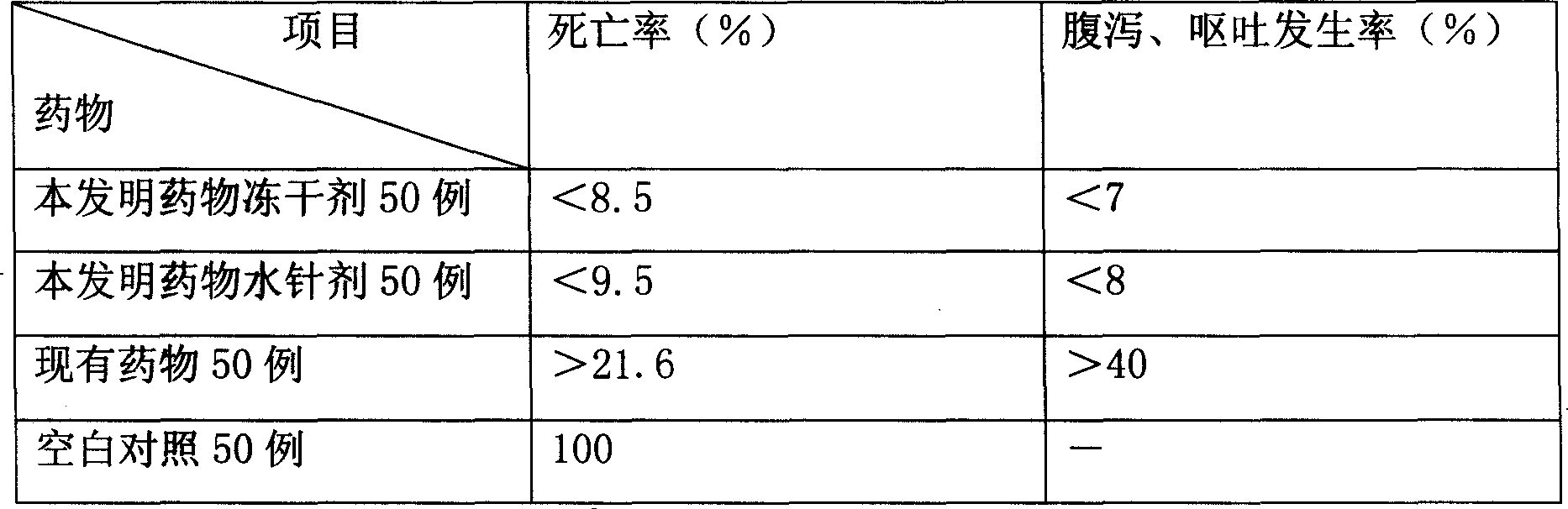
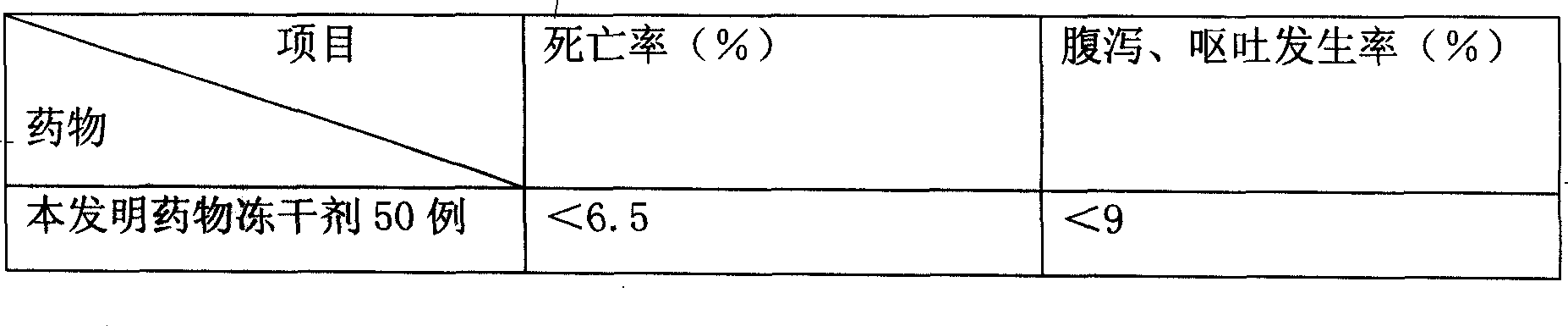
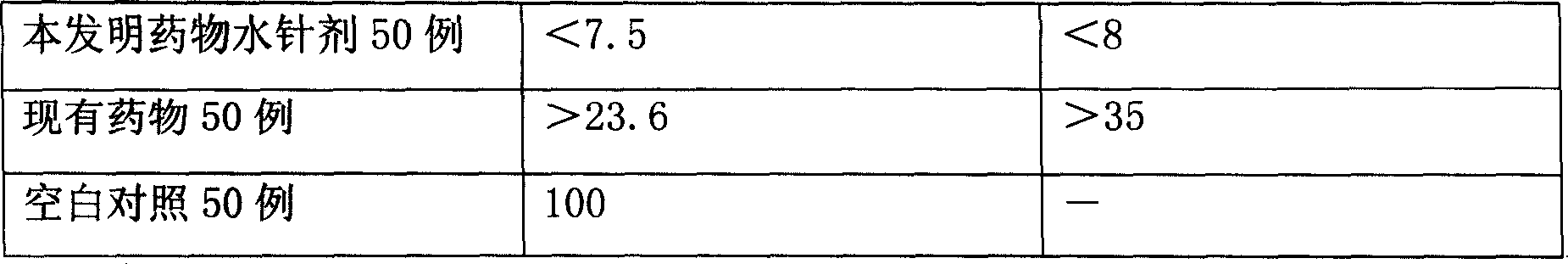
[0078] Pharmacodynamic verification test:
[0079] Infect the mouse model with pn...
Embodiment 3
[0082] The formula that present embodiment adopts is as follows:
[0083] Soy lecithin and dipalmitoylcholine mixture 4:1) 0.2mol
[0084] Cholesterol and β-sitosterol mixture (2:1) 0.2mol
[0085] Clindamycin Phosphate and Levofloxacin Lactate Random Ratio Mixture 600mg
[0086] Reduced glutathione and tiopronin mixture (molar ratio 1:4) 30g
[0087] Polyethylene glycol 6000 0.02mol
[0088] Sodium glutamate 50g
[0089] The number of grams contained in the mixture of Pluronic F68 and hypromellose in any ratio in 100ml liquid medicine is 0.2%-0.7%g / ml
[0090] Phosphate buffer 0.015M pH3.0-5.5 added to 1000ml
[0091] The 200 mg of the arbitrary ratio mixture of clindamycin phosphate and levofloxacin lactate is calculated based on the amount of clindamycin and levofloxacin.
[0092] Preparation steps: see Example 1.
[0093] Pharmacodynamic verification test:
[0094] Infect the mouse model with pneumococcus, and inject it via intravenous injection 6 hours after infec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com