Technique for preparing diacerein by two-step oxidation process
A technology of diacerein and oxidation synthesis, which is applied in the direction of oxidative preparation of carboxylic acid, organic compound preparation, carboxylate preparation, etc., which can solve the problems of complex separation and purification of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
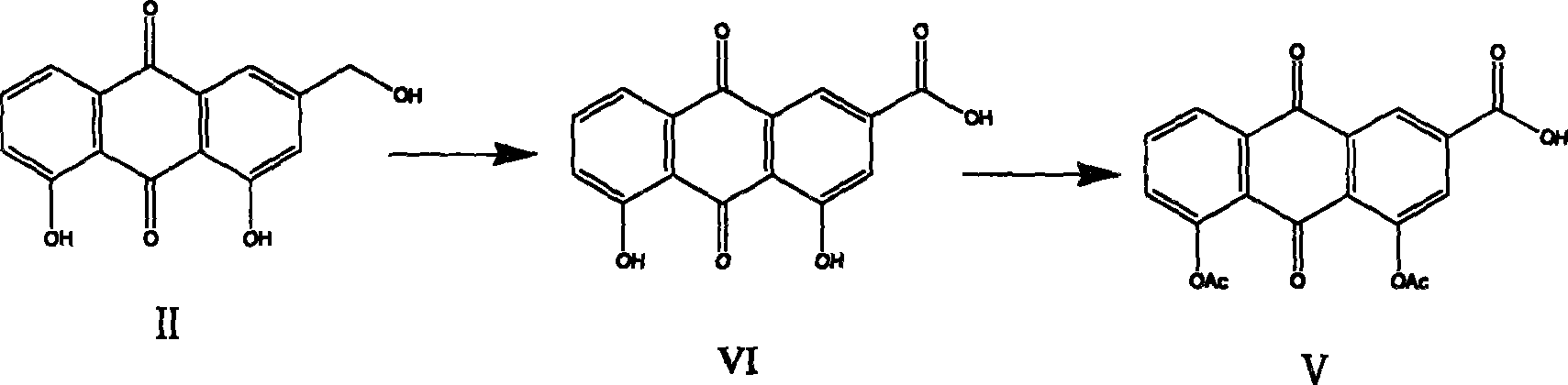
[0056] Example 1: Put 100 grams of 90% aloin into a 2-liter four-necked bottle, then add 800 milliliters of aqueous solution of 924 grams of ferric chloride, heat up to 98°C under stirring, keep the temperature for 7 hours, cool naturally to room temperature, and filter , the obtained filter cake was washed with water, and dried to obtain 80 grams of aloe-emodin crude product. The above crude product was repeatedly extracted with toluene, the combined extracts were concentrated and the resulting residue was recrystallized with 200 ml of acetic acid to obtain 45.9 g of aloe-emodin with a melting point of 216-218°C.
Embodiment 2
[0057] Example 2: 100 grams of 90% aloin was put into a 2-liter four-necked bottle, and then 1000 milliliters of aqueous solution of 1000 grams of ferric chloride was added, and the temperature was raised to 80° C. under stirring, and the reaction was kept for 10 hours, cooled naturally to room temperature, and filtered , the resulting filter cake was washed with water, and dried to obtain 85 grams of aloe-emodin crude product. The above crude product was repeatedly extracted with toluene, the combined extracts were concentrated and the resulting residue was recrystallized with 200 ml of acetic acid to obtain 50 g of aloe-emodin with a melting point of 216-218°C.
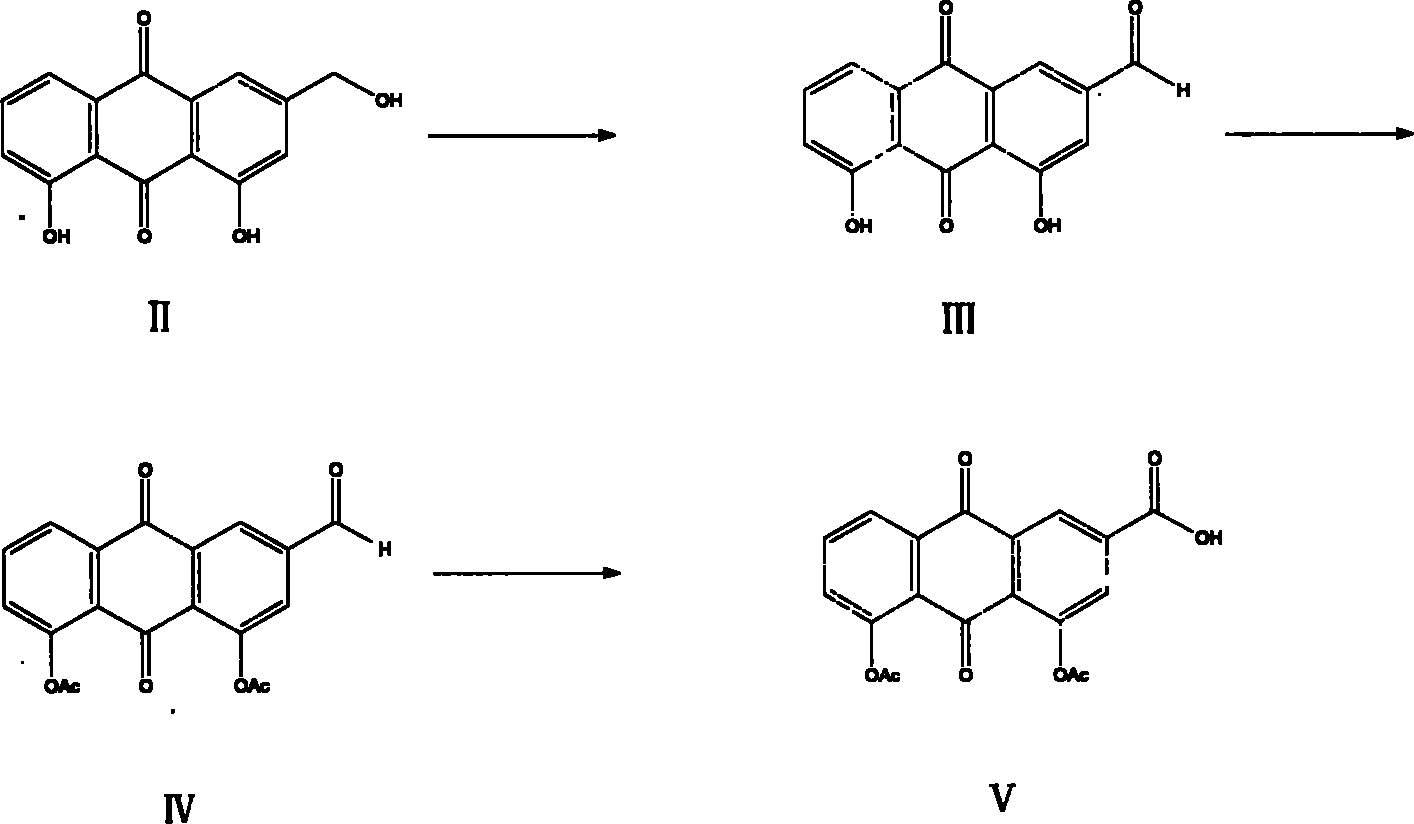
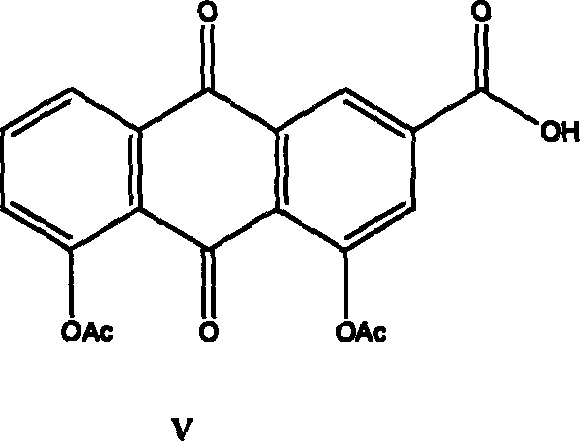
[0058] (2) Preparation of rhein III
[0059] Embodiment 1: 10 grams of aloe-emodin were put into a 1000 milliliter four-neck flask equipped with 40 grams of PCC with a stirrer and a reflux condenser, 800 milliliters of dichloroethane was added, and the reflux reaction was carried out under vigorous stirring for 10 h...
Embodiment 3
[0061] Example 3: Put 10 grams of aloe-emodin into a 1000 ml four-neck flask equipped with 40 grams of manganese dioxide with a stirrer and a reflux condenser, add 1000 ml of acetone, and reflux for 20 hours under vigorous stirring. Concentrate by filtration to obtain 5.8 g of rhein III.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com