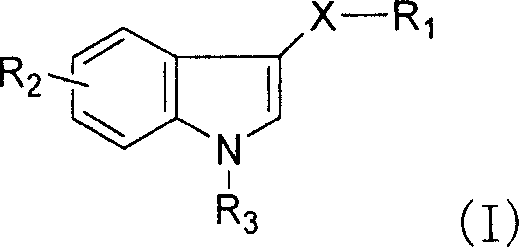
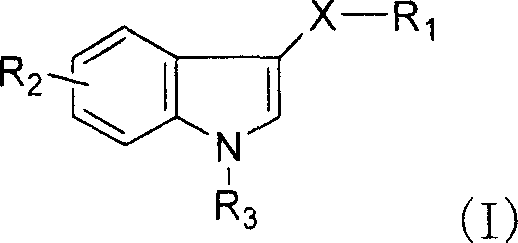
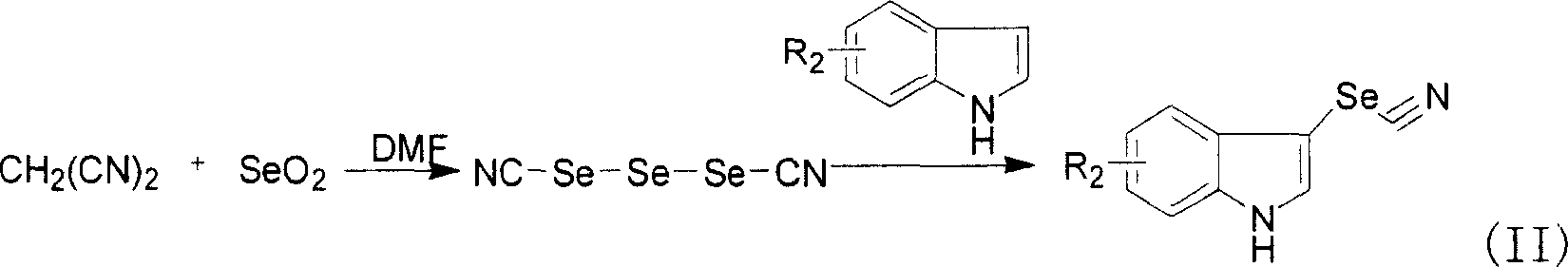Synthesis of novel 3-seleno indole with vegetation promotion and selenium enriching functions
A selenium generation and compound technology, applied in the direction of plant growth regulators, plant growth regulators, botanical equipment and methods, etc., can solve the problems of not being able to increase agricultural production, limiting the scope of application, and lacking some functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Synthesis of 3-diselenyl-1-hydrogen-indole (compound 1)
[0062]
[0063] Dissolve 2 grams (30 mmol) of malononitrile in 5 ml of DMF, add 6.7 grams (60 mmol) of selenium dioxide in batches under vigorous stirring, and absorb the released gas with 5% NaOH solution. (22.5mmol) DMF (5ml) solution of indole, after the dropwise addition, stir at room temperature until the disappearance of indole, about 3 hours. The reaction solution was poured into ice water and kept stirring, a yellow solid precipitated, suction filtered, the solid was taken and dried, and recrystallized from toluene to obtain 3.9 g of the product, the yield was 78%, the melting point was 75-79°C, GC / MS: 222, 196, 169, 42, 117, 89.
Embodiment 2
[0064] Embodiment 2: Synthesis of 3-diselenyl-1-hydrogen-indole (compound 2)
[0065]
[0066] (2) Weigh 2.22 grams (10 mmol) of the product synthesized in step (1), add it to 10 ml of absolute ethanol, add 0.2 grams (5 mmol) of NaOH in 1 ml of aqueous solution dropwise under N protection conditions, stir at room temperature for 30 minutes, then add 10 ml of water Hydrolysis, extraction twice with 20 ml of ether, drying of the organic phase over anhydrous magnesium sulfate, rotary evaporation under reduced pressure to obtain 1.6 g of the product with a yield of 82%.
[0067] Melting point 122-124°C; 1 H NMR (CDCl 3)δ (ppm): 7.47 (d, J=8.1, 2H, indole-CH), 7.44 (d, J=8.0, 2H, indole-CH), 7.29 (s, 2H, indole-CH), 7.18(t, 2H, indole-CH), 7.06(t, 2H, indole-CH); IR(KBr): 3339, 2956, 1477, 1444, 1401, 1099cm -1 ; HR-MS: C 16 h 13 N 2 Se 2 (M+H) + Calculated value: 392.9409; Tested value: 392.9394.
Embodiment 3
[0068] Embodiment 3: Synthesis of selenium-acetate ethyl-3-selenium-indole (compound 3)
[0069]
[0070] Weigh 0.392 g (1 mmol) of compound 2, dissolve it in 5 ml of ethanol, and stir under nitrogen protection. Add 0.41 g NaBH in batches 4 , stirred for about 30 minutes, the solution changed from yellow to colorless, and a solution of 0.117 g of ethyl bromoacetate in 5 ml of ethanol was added dropwise. TLC detects that the reaction is complete. Spin to remove ethanol. Add 10ml ethyl acetate to dissolve and wash twice with water. The organic phase was taken, dried, concentrated and then subjected to silica gel column chromatography to obtain the product.
[0071] The melting point is 65-66°C.
[0072] 1 H NMR (CDCl 3 )6 (ppm) 7.62 (d, 1H, J=7.65, indole-H), 7.52 (s, 1H, indole-H), 7.45 (d, 1H, J=4.99, indole-H), 7.18 -7.10(m, 2H, indole-H), 3.95(s, 2H, -CH 2 -), 3.36 (m, 2H, -CH 2 -), 2.87(t, 3H, -CH 3 ).
[0073] IR (KBr): 3263, 2918, 1707, 1410, 1257, 1080cm ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com