Method for preparing 3-pyridine acetic acid hydrochloride
A technology of pyridine acetic acid hydrochloride and pyridine thioacetyl, which is applied in the synthesis field of pharmaceutical intermediate 3-pyridine acetic acid hydrochloride, can solve the problems of metallic sodium such as danger, difficulty in storage, complicated post-processing, etc., and achieve The effect of shortening the preparation process, easy operation and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
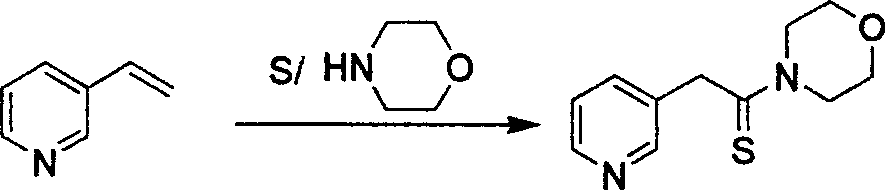
[0021] a) the preparation steps of 3-pyridinethioacetylmorpholine
[0022] Add 86.8g of 3-vinylpyridine to 79g of morpholine, add 29g of sulfur while stirring, heat and reflux for 12 hours, pour the reactant into ice water and filter, wash the crystals with ice water, and dry naturally in the air to obtain light yellow 160.2 g of crystals, yield 87.3%.
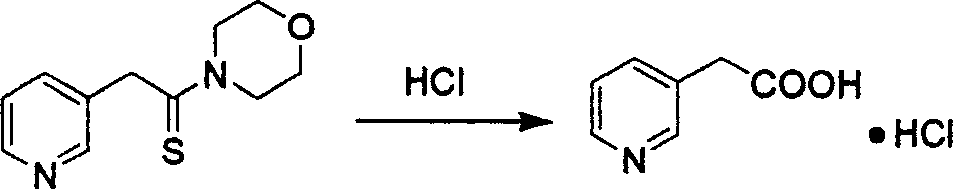
[0023] b) Preparation steps of 3-pyridine acetic acid hydrochloride
[0024] Mix 160.2g of 3-pyridinethioacetylmorpholine with 182ml of hydrochloric acid, heat to reflux for 6 hours, filter, concentrate under reduced pressure, cool and crystallize, refine with 130ml of concentrated hydrochloric acid, and dry to obtain 107.6g of white crystal 3-pyridineacetic acid hydrochloride , yield 86%.
Embodiment 2
[0026] a) the preparation steps of 3-pyridinethioacetylmorpholine
[0027] Add 86.8g of 3-vinylpyridine to 79g of morpholine, add 29g of sulfur while stirring, heat and reflux for 14 hours, pour the reactant into ice water and filter, wash the crystals with ice water, and dry naturally in the air to obtain light yellow Crystallization 165g, yield 90%.
[0028] b) Preparation steps of 3-pyridine acetic acid hydrochloride
[0029] 160.2 g of 3-pyridinethioacetylmorpholine was mixed with 182 ml of hydrochloric acid, heated to reflux for 5 hours, filtered, concentrated under reduced pressure, cooled and crystallized, refined with 110 ml of concentrated hydrochloric acid, and dried to obtain 110 g of white crystal 3-pyridine acetate hydrochloride, Yield 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com