Technique for synthesizing ester amine and quaternary ammonium salt thereof
A technology of quaternary ammonium salts and ester amines, which is applied in the technical field of synthesizing ester amines and quaternary ammonium salts, to reduce production costs, reduce nitrogen consumption, and ensure product color.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
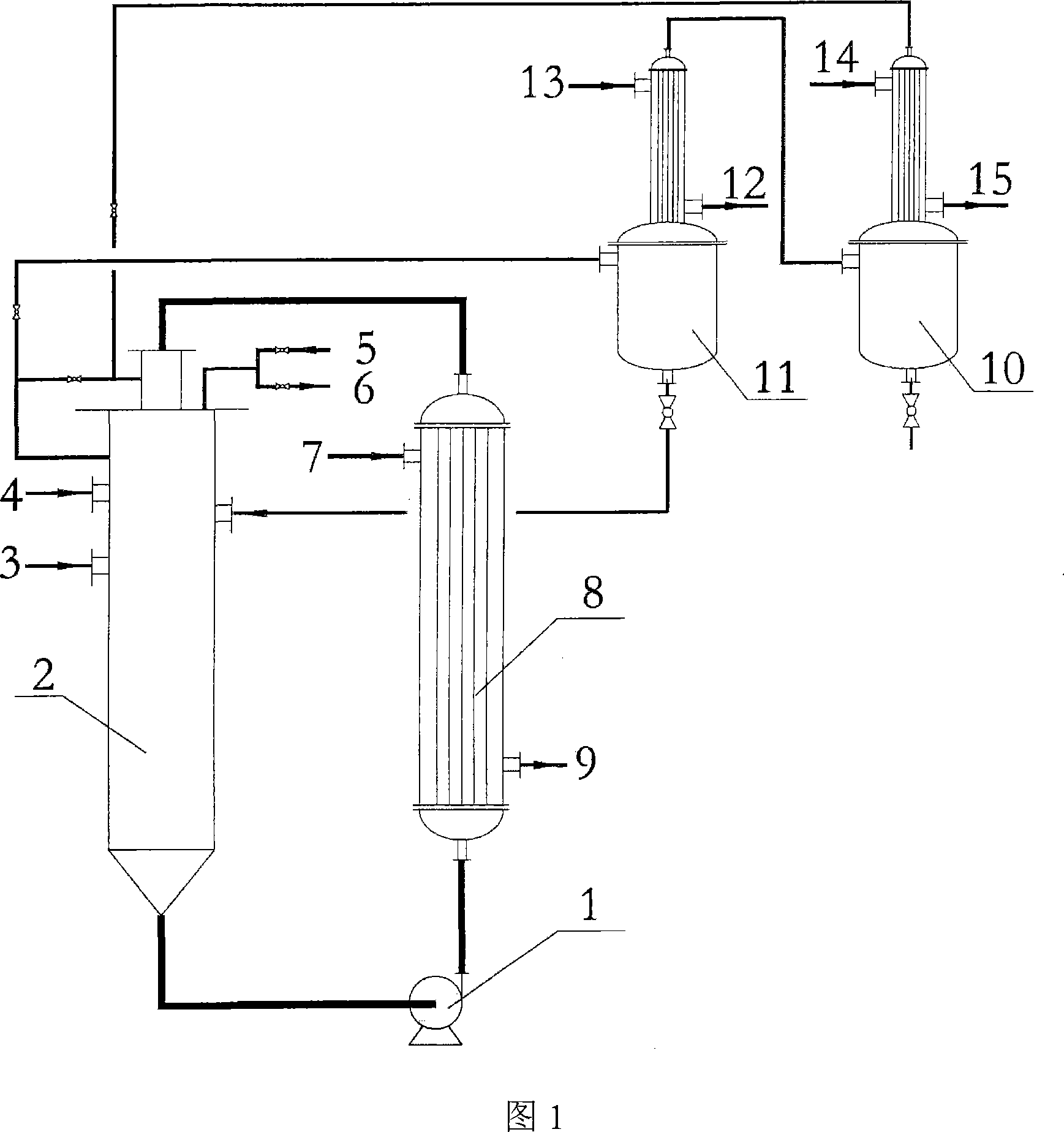
[0021] Ester amination reaction: Weigh 57Kg of molten stearic acid, 14.9Kg of triethanolamine and 200g of alkylbenzenesulfonic acid and add them to 200L loop reactor 2, replace with nitrogen, and keep the entire loop system at a reaction pressure of 0.2MPa At the nitrogen pressure, the external circulation pump 1 was started, and the external circulation heat exchanger 8 and the loop reactor 2 were used to raise the temperature of the material to the reaction temperature of 180 °C, and the reaction was started. After 6 hours of reaction time, when the acid value of the ester amine product was measured to be 2.8 mgKOH / g, the reaction was terminated to obtain the ester amine.
[0022] Quaternization reaction: use the external circulation heat exchanger 8 and the loop reactor 2 to reduce the temperature of the ester amine to 70°C of the reaction temperature, the system pressure to normal pressure, and add 10Kg of isopropanol to the loop reactor through the material inlet 4 Then, ...
Embodiment 2
[0024] Ester amination reaction: melted stearic acid 100Kg, methyldiethanolamine 20.9Kg, phosphorous acid 100g, reaction pressure 0.5MPa, reaction temperature 170°C, reaction time 5 hours, product acid value 3mgKOH / g.
[0025] Quaternization reaction: the reaction temperature is 60°C, the amount of isopropanol is 14.2Kg, the amount of dimethyl sulfate is 22Kg, the feeding time is 2 hours, and after the reaction time is 6 hours, the free amine content in the product is 0.016mmol / g. Other conditions are the same as in Example 1.
Embodiment 3
[0027] Ester amination reaction: 56.4Kg of oleic acid, 14Kg of triethanolamine, 200g of alkylbenzenesulfonic acid, reaction pressure 0.4MPa, reaction temperature 200℃, reaction time 4 hours, product acid value 2.5mgKOH / g.
[0028] Quaternization reaction: the reaction temperature is 80°C, the amount of isopropanol is 10Kg, the amount of methyl chloride is 5.3Kg, the feeding time is 1 hour, and after the reaction time is 5 hours, the free amine content in the product is 0.015mmol / g. Other conditions are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com