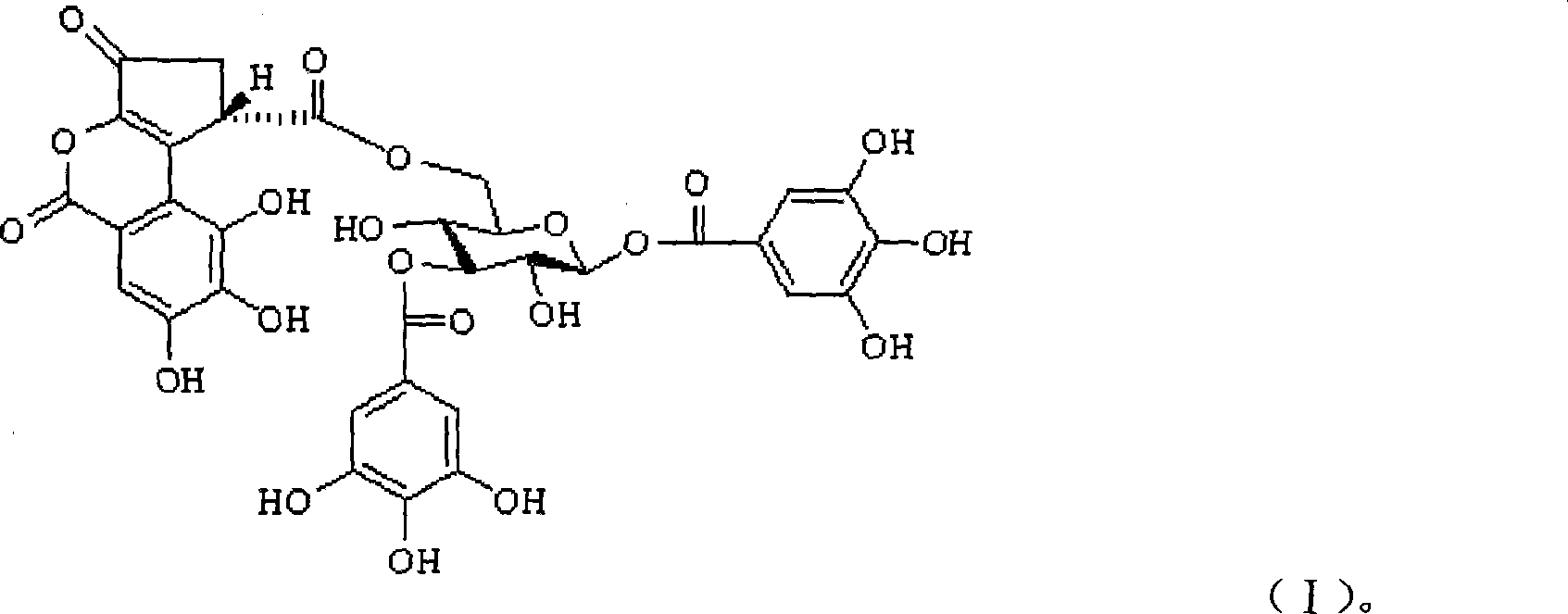
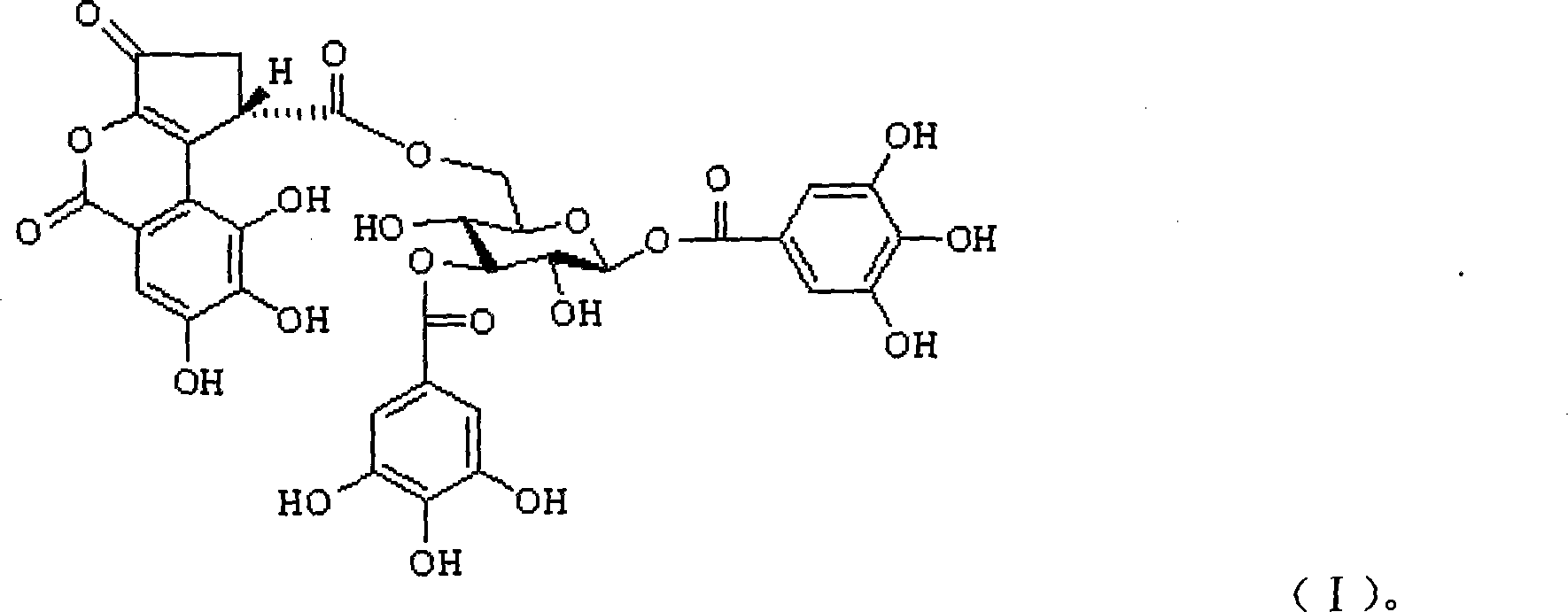
1,3-O-di-galloyl-6-O-(S)-decapetalous caesalpinia acyl-beta-D-glucopyranose and application thereof
A galloyl and glucopyranose technology, applied in the direction of sugar derivatives, sugar derivatives, esterified saccharides, etc., can solve the problems of not disclosing the use of such compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
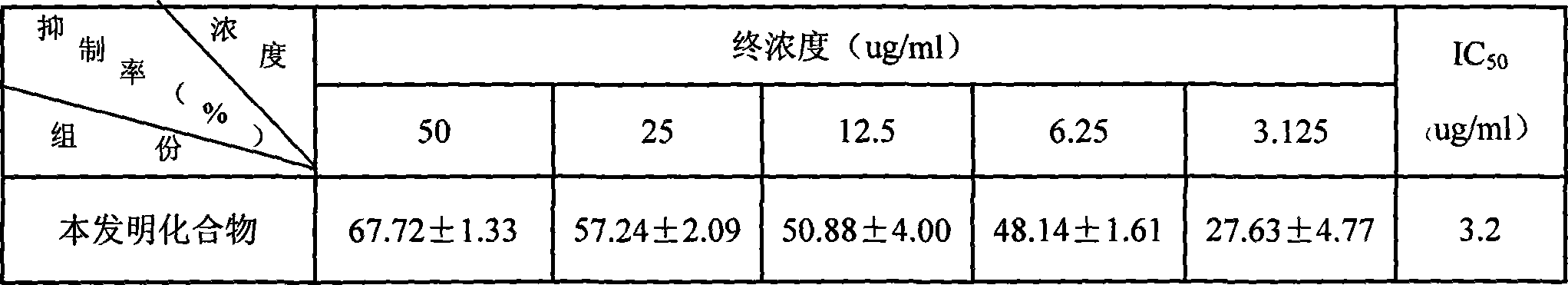
[0039] 1. Take 1.73kg of Japanese snake mushroom, chop it, soak it with 70% methanol at room temperature, filter it overnight, and concentrate the filtrate under reduced pressure with a rotary evaporator to obtain 223.7g of the total extract; dilute the extract with water and dilute it with ether Extraction, take the water phase, and then extract with ethyl acetate to obtain the dissolved part of ethyl acetate; pass the dissolved part of ethyl acetate through Chromatorex ODS, elute with water, and then elute with 10-80% methanol, collect 40-80% methanol to wash Remove liquid, recover methanol under reduced pressure, add a little methanol to dissolve the residue; then apply MCI-gel CHP20P, elute with water and then 10-80% methanol, collect 40-80% methanol eluate, recover methanol under reduced pressure, The residue was dissolved by adding a little methanol; then it was chromatographed on Sephadex LH-20, eluted with water and then eluted with 10% to 80% methanol, and 50% to 80% o...
example 1
[0057] 【prescription】
[0058] 1,3-O-Di-Galloyl-6-O-(S)-Celoyl-β-D-Glucopyranose 20g
[0059] Microcrystalline Cellulose 48g
[0060] Soluble starch 30g
[0062] A total of 1000 tablets were produced, each containing 20 mg of the compound.
[0063] 【Preparation】
[0064] Weigh 20g of the compound, add soluble starch to dilute to 50g, mix well, then weigh 48g of microcrystalline cellulose, use 95% ethanol to make soft material, sieve and granulate, dry at low temperature at 50°C, granulate, add talc powder 2g , mixed evenly, compressed into tablets (0.1g each), inspected for quality, packaged, and obtained.
[0065] 【Dosage】
[0066] 4 tablets each time, 3 times a day.
[0067] 【For people】
[0068] Applicable to various tumor patients.
example 2
[0070] 【prescription】
[0071] 1,3-O-Di-Galloyl-6-O-(S)-Celoyl-β-D-Glucopyranose 10g
[0072] Microcrystalline Cellulose 48g
[0073] Soluble starch 40g
[0074] Talc powder 2g
[0075] A total of 1000 tablets were produced, each containing 20 mg of the compound.
[0076] 【Preparation】
[0077] Weigh 10g of the compound, add soluble starch to dilute to 50g, mix well, then weigh 48g of microcrystalline cellulose, use 95% ethanol to make soft material, sieve and granulate, dry at low temperature at 50°C, granulate, add talc powder 2g , mixed evenly, compressed into tablets (0.1g each), inspected for quality, packaged, and obtained.
[0078] 【Dosage】
[0079] 4 tablets each time, 3 times a day.
[0080] 【For people】
[0081] Applicable to various tumor patients.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com