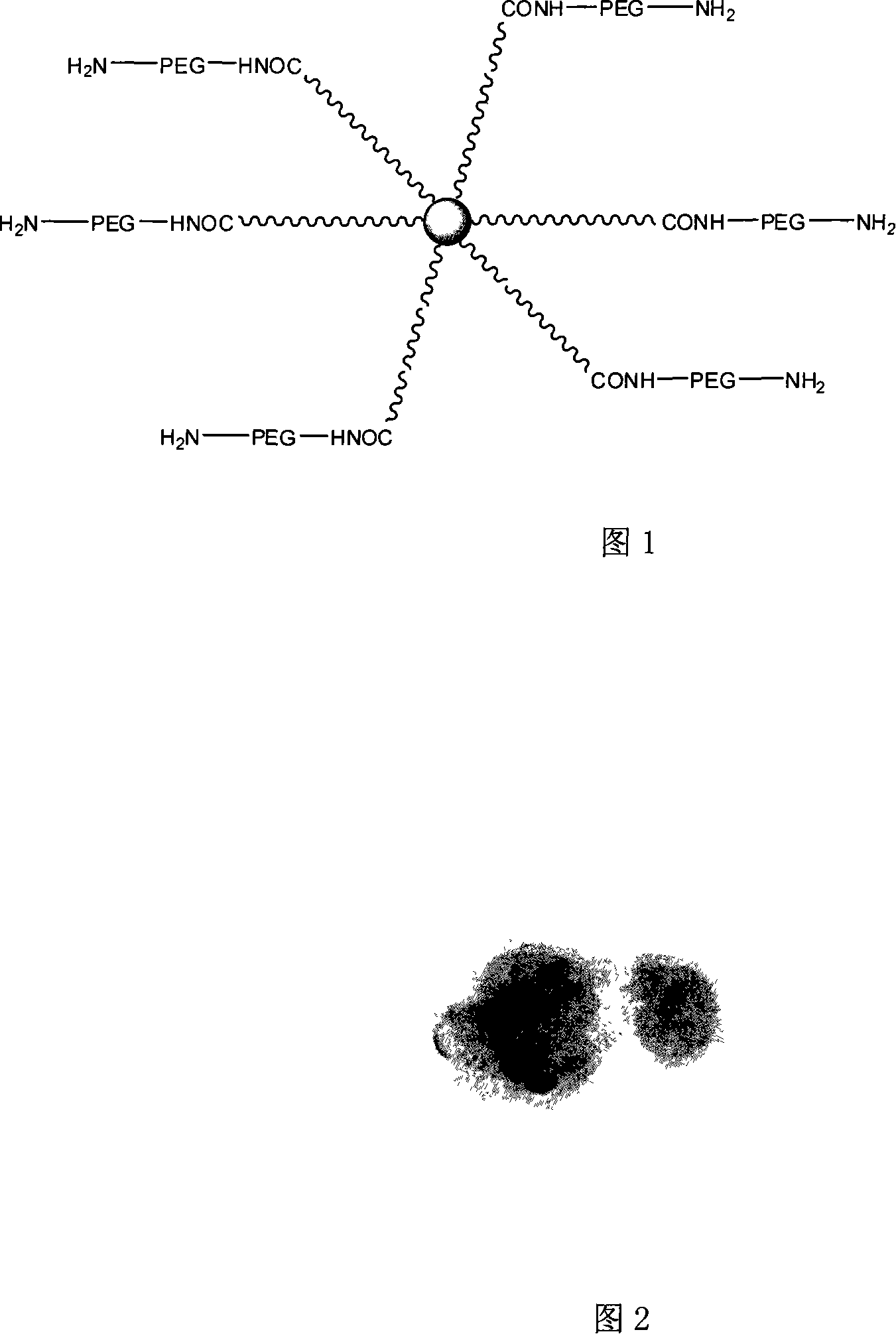
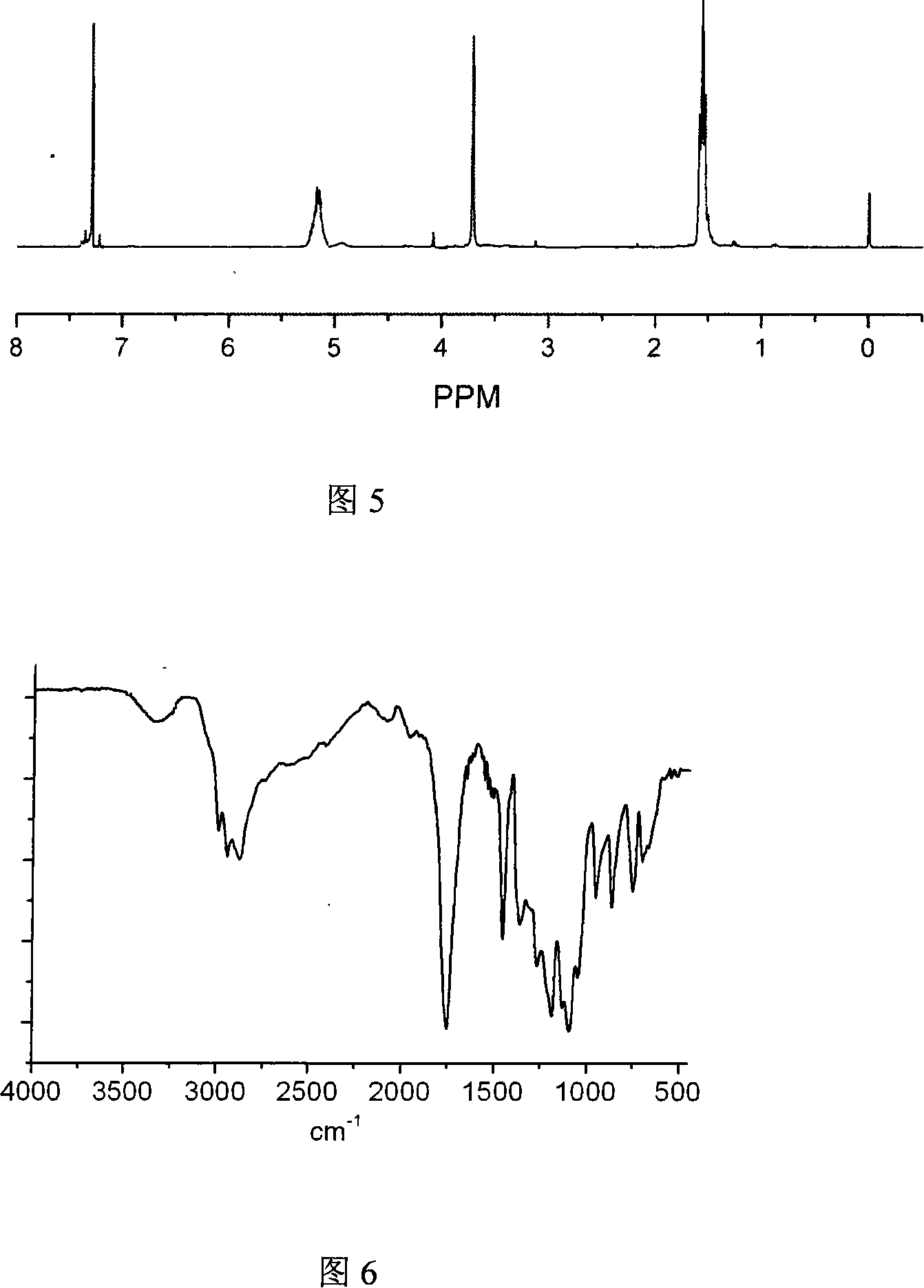
Terminal amido group start-type poly (lactic-co-glycolic acid)/polyglycol block copolymer, preparation method, medicament-carried nano micelle and application
A polyglycolide and block copolymer technology, which can be applied in the directions of ether/acetal active ingredients, antitumor drugs, drug combinations, etc., can solve the problem of less modifiable end groups and low drug encapsulation efficiency. and other problems, to achieve the effect of inhibiting tumor angiogenesis and improving the inhibition rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A kind of amine-terminated star polylactide / polyethylene glycol block copolymer is made by following method:
[0047] (1) Add glycolide and lactide with a molar ratio of 1:5 to the polymerization sealed tube, and then add polyamide-amine-OH with a molar ratio of 1:50 to the monomer Dendritic macromolecule constitutes reactant, adds stannous octoate as catalyst, and the addition mass of described catalyst is 0.1% of described reactant mass, vacuumizes nitrogen replacement 3 times, is 40Pa sealing tube at vacuum degree, at 135 ℃, React for 48 hours to obtain hydroxyl-terminated star polylactide;
[0048] (2) The hydroxyl-terminated star-shaped polyglycolide and succinic anhydride are prepared according to the molar ratio of the hydroxyl group in the hydroxyl-terminated star-shaped polyglycolide molecule to succinic anhydride of 1:14, at 25°C , and reacted for 30 hours to obtain carboxyl-terminated star polylactide;
[0049] (3) According to the mass ratio of 1:20, the c...
Embodiment 2
[0054] A kind of amine-terminated star polylactide / polyethylene glycol block copolymer is made by following method:
[0055] (1) Add glycolide and lactide with a molar ratio of 1:1 to the polymerization sealed tube, then add inositol with a molar ratio of 1:30 to form the reactant, add Stannous octoate is the catalyst, the added mass of the catalyst is 0.1% of the mass of the reactant, evacuated and replaced by nitrogen twice, sealed at a vacuum of 80Pa, and reacted for 60 hours at 110°C to obtain a star-shaped hydroxyl group. Polyethylene lactide;
[0056] (2) The hydroxyl-terminated star polylactide and succinic anhydride are prepared at 20° C. according to the molar ratio of the hydroxyl group in the hydroxyl-terminated star polylactide molecule to succinic anhydride of 1:15. , and reacted for 40 hours to obtain carboxyl-terminated star polylactide;
[0057] (3) According to the mass ratio of 1:10, the carboxyl-terminated star polylactide is dissolved in anhydrous toluene...
Embodiment 3
[0059] A kind of amine-terminated star polylactide / polyethylene glycol block copolymer is made by following method:
[0060] (1) Add glycolide and lactide with a molar ratio of 1:10 to the polymerization sealed tube, and then add glycerol with a molar ratio of 1:100 to form the reactant, Add stannous octoate as a catalyst, the mass of the catalyst added is 0.1% of the mass of the reactant, evacuate nitrogen for 4 times, seal the tube at a vacuum degree of 5Pa, and react at 150°C for 24 hours to obtain the hydroxyl-terminated star Type polyethylene lactide;
[0061] (2) The hydroxyl-terminated star-shaped polyglycolide and succinic anhydride are prepared according to the molar ratio of the hydroxyl group in the hydroxyl-terminated star-shaped polyglycolide molecule to succinic anhydride as 1:9, at 30°C , and reacted for 20 hours to obtain carboxyl-terminated star polylactide;
[0062] (3) According to the mass ratio of 1:30, the carboxyl-terminated star polylactide is dissolv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com