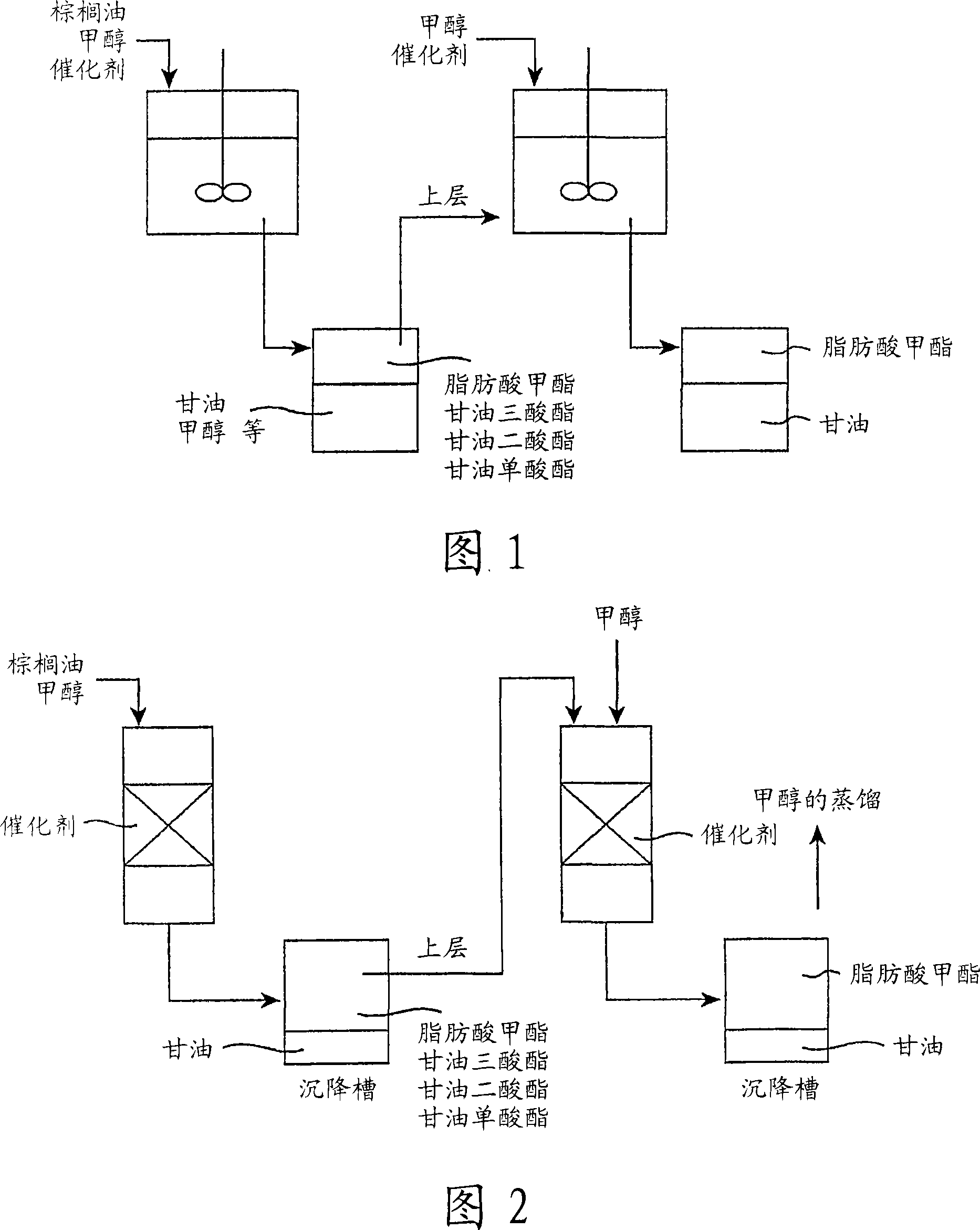
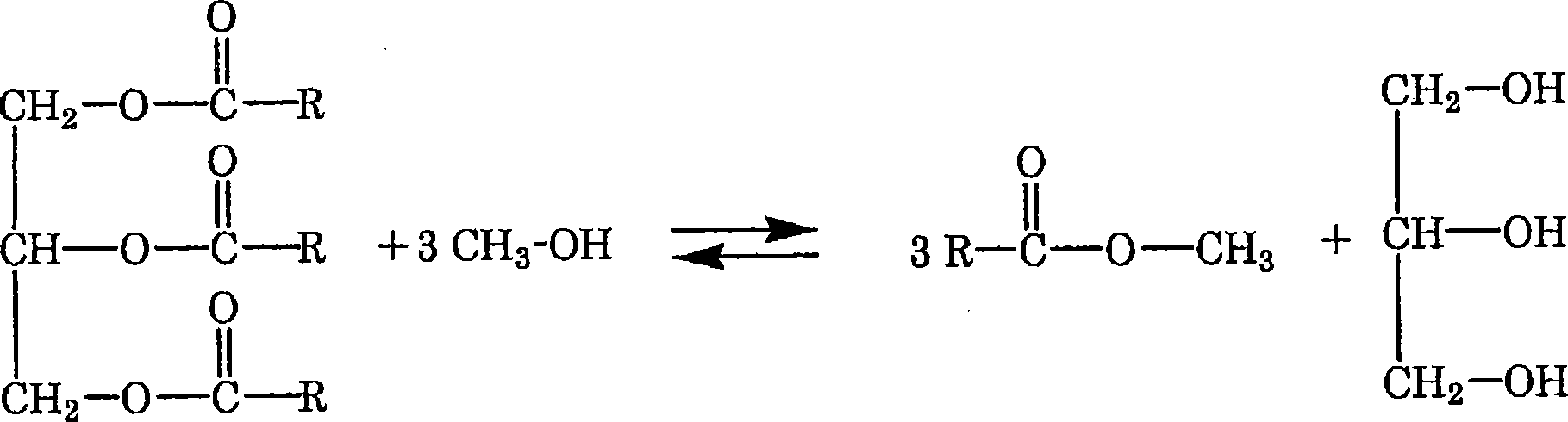
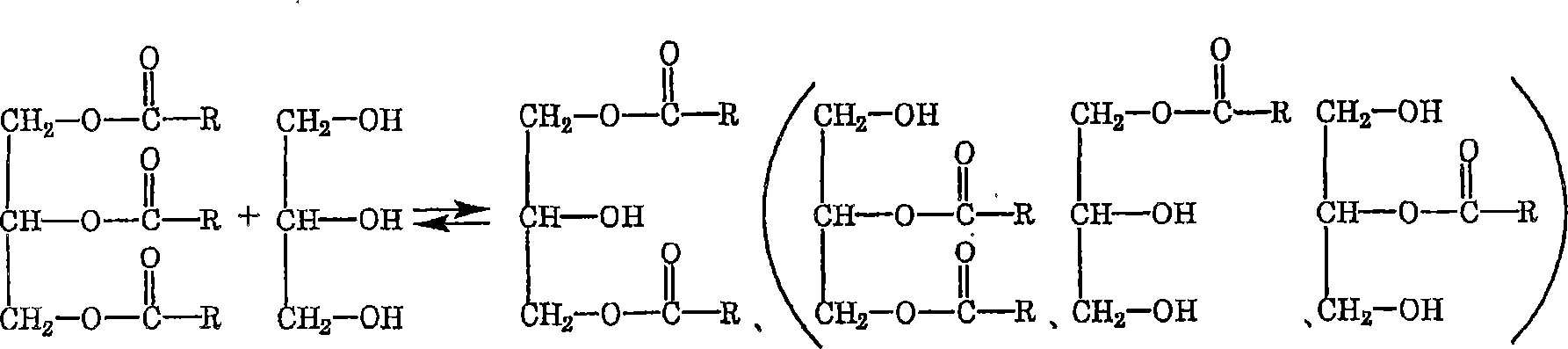
Method for producing fatty acid alkyl esters and/or glycerin
A technology of fatty acid alkyl ester and production method, which is applied in the directions of fatty acid esterification, organic chemical method, ester group and hydroxyl reaction preparation, etc., can solve the problems of shortened catalyst life, complicated separation and purification steps, leaching of metal components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0140]The present invention will be described in more detail below with reference to Examples, but the present invention is not limited to these Examples.
[0141] Conversion and yield were calculated according to the following formula.
[0142] (Conversion rate (%))=(the mole number of fat or oil consumed when the reaction is completed) / (feeding mole number of fat or oil)×100(%)
[0143] (the productive rate of methyl ester (mol%))={(the molar number of the methyl ester that makes when reaction completes) / (feeding molar number of effective fatty acid)}×100(%)
[0144] (the productive rate of diglyceride (mol%))={[(the mole number of diglyceride produced when the reaction is completed)×2] / (the feed mole number of effective fatty acid)}×100(%)
[0145] (the productive rate (mol%) of monoglyceride)={(the mole number of monoglyceride when the reaction is completed) / (feeding mole number of effective fatty acid)}×100(%)
[0146] (the productive rate of glycerol (mol %))={(the mol...
preparation example 1
[0150] Catalyst preparation example 1: MnTiO 3 Catalyst preparation
[0151] 200g manganese carbonate (MnCO 3 ) and 150g anatase titanium dioxide (TiO 2 ) were mixed in powder form, and the mixture was calcined at 1000° C. for 5 hours in an air stream to obtain 273 g of MnTiO 3 catalyst. XRD analysis of the catalyst showed that the catalyst has an ilmenite structure and contains a small amount of rutile TiO 2 .
preparation example 2
[0152] Catalyst preparation example 2: ZnTiO 3 Preparation of (Anatase) Catalyst
[0153] 20g of zinc oxide (ZnO) and 20g of anatase titanium dioxide (TiO 2 ) mixed in powder form. The mixture was calcined at 700 °C for 4 hours in air flow to obtain ZnTiO 3 catalyst. XRD analysis of the catalyst shows that the catalyst is ZnTiO with ilmenite structure 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com