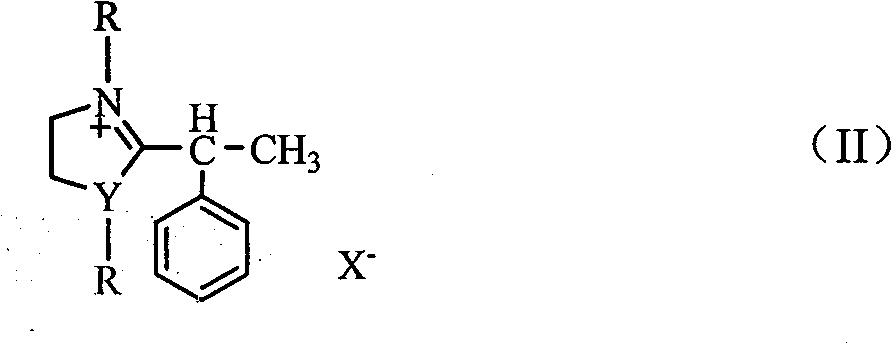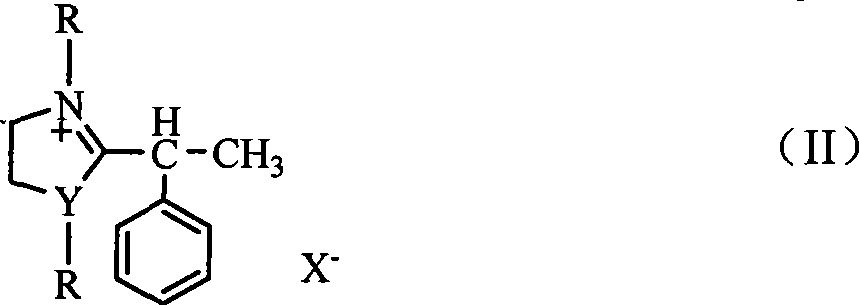Method for synthesizing black nightshade aldehyde
A synthetic method and the technology of solanum aldehyde, which are applied in the field of synthesis of solanum aldehyde, can solve the problems of high cost and unfavorable industrial production, and achieve the effects of low cost, simple reduction method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
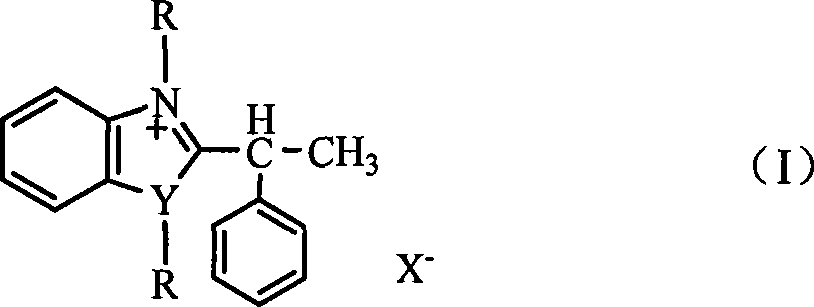
[0017] 2-(α-phenylethyl)benzimidazole methyl iodide salt was synthesized with reference to literature [Craig, J.C.; Erwuibe, N.N.Synthesis, 1981, 4, 303.]. 2-(α-phenylethyl)benzimidazole (synthesized from o-phenylenediamine and α-phenylpropionic acid, reference literature: Shi Zhen, Gu Huan. Chinese Science (B Series), 1996, 26 (5), 403.) under the action of sodium alkoxide, react with methyl iodide at reflux temperature for 18h, ethanol recrystallization promptly obtains 2-(alpha-phenylethyl) benzimidazole methyl iodide salt, productive rate 85%, Elemental analysis, calculated value (%): C53.97, H5.03, N 7.41; found value (%): C 53.94, H 5.09, N 7.38; MS m / z: 251 (M + -I).
[0018] Under stirring, add 0.01mol 2-(α-phenylethyl)benzimidazole methyl iodide salt into 20mL ethanol solution, after adding and dissolving, lower the temperature to 0-5°C, at this temperature, Add 0.02mol sodium chips in batches, keep the temperature and stir for 0.5h, then slowly raise the temperatur...
Embodiment 2
[0020] 2-(α-phenylethyl)benzoxazole ethyl bromide was synthesized with reference to Example 1.
[0021] Under stirring, add 0.01mol 2-(α-phenylethyl)benzoxazolyl ethyl bromide into 30mL ethanol solution, after adding and dissolving, lower the temperature to 0-5°C, at this temperature , add 0.02mol sodium chips in batches, keep the temperature and stir the reaction for 0.5h, slowly raise the temperature to room temperature and then stir the reaction for 0.5h, add 0.01mol sodium chips, and then reflux for 1.5 hours, add 5% hydrochloric acid aqueous solution, make the reaction The mixture was kept at a pH of 3-5 and a temperature of 50-60° C., stirred for 1.5 h, extracted with chloroform (5×30 mL), and evaporated to obtain solanaldehyde. The product is a colorless liquid with a yield of 68%. The NMR, mass spectrometry, infrared and elemental analysis data are consistent with those reported in the literature.
Embodiment 3
[0023] 2-(α-phenylethyl)benzimidazole benzyl chloride was synthesized with reference to Example 1.
[0024] Under stirring, add 0.01mol 2-(α-phenylethyl)benzimidazole benzyl chloride salt into 30mL ethanol solution, after adding and dissolving, lower the temperature to 0-5°C, at this temperature, Add 0.02mol sodium chips in batches, keep the temperature and stir for 0.5h, then slowly raise the temperature to room temperature and then stir for 0.5h, add 0.01mol sodium chips, and then reflux for 1.5 hours, add 5% aqueous hydrochloric acid to make the reaction mixture Keeping the pH value at 3-5 and the temperature at 50-60° C., stir the reaction for 1.5 h, extract with chloroform (5×30 mL), and distill off the solvent to obtain solanin. The product is a colorless liquid with a yield of 62%. The NMR, mass spectrometry, infrared and elemental analysis data are consistent with those reported in the literature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com