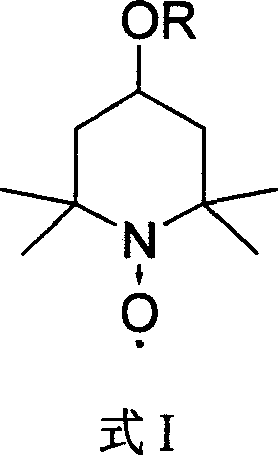
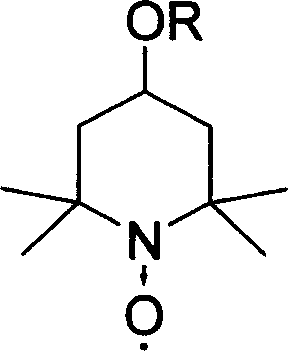
Catalyst for selective oxidation of protection monosaccharide primary hydroxy group
A technology of sugar primary hydroxyl and selectivity, which is applied in the field of catalysts for the selective oxidation of monosaccharide primary hydroxyl, can solve the problems of harsh reaction conditions, many by-products, narrow pH range, etc., and achieve relaxed reaction conditions, fast reaction speed, The effect of fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of 4-tert-butoxy-2,2,6,6-tetramethylpiperidine-N-oxide
[0032] At 0°C, add 300ml of dichloromethane, 60ml of pyridine, 30g of 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxide into a 1L three-necked flask, and then dropwise add 66g of p-toluenesulfonate A solution of acid chloride dissolved in 200ml of dichloromethane was reacted for 180 minutes. Then, a solution of 50 g of sodium tert-butoxide dissolved in 200 ml of dichloromethane was added dropwise to the solution, and the reaction was stirred for 120 minutes. After the reaction is complete, wash the reaction solution with water three times, dry the organic layer with anhydrous sodium sulfate, evaporate dichloromethane to dryness, add 100ml of n-hexane, freeze and crystallize, and filter to obtain 4-tert-butoxy-2,2,6,6- 34 g of red crystals of tetramethylpiperidine-N-oxide. 1 H NMR (CDCl 3 , 300MHz, δ) 1.22 (s, 9H) 1.73 (s, 12H) 1.95-2.20 (m, 4H) 2.83 (m, 1H); Elemental analysis: C, 68.36; H, ...
Embodiment 2
[0033] Example 2 Preparation of 4-isopropoxy-2,2,6,6-tetramethylpiperidine-N-oxide
[0034] At 0°C, add 300ml of dichloromethane, 60ml of pyridine, 30g of 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxide into a 1L three-necked flask, and then dropwise add 66g of p-toluenesulfonate A solution of acid chloride dissolved in 200ml of dichloromethane was reacted for 180 minutes. Then, a solution of 46.5 g of sodium isopropoxide dissolved in 200 ml of dichloromethane was added dropwise to the solution, and the reaction was stirred for 120 minutes. After the reaction is complete, wash the reaction solution with water three times, dry the organic layer with anhydrous sodium sulfate, evaporate dichloromethane to dryness, add 100ml of n-hexane, freeze and crystallize, and filter to obtain 4-isopropoxy-2,2,6,6- 28 g of red crystals of tetramethylpiperidine-N-oxide. 1 H NMR (CDCl 3 , 300MHz, δ) 1.17 (s, 6H) 1.71 (s, 12H) 1.95-2.20 (m, 4H) 2.83 (m, 1H) 3.20 (m, 1H); elemental analysis: C...
Embodiment 3
[0035] Example 3 Preparation of 4-cyclohexyloxy-2,2,6,6-tetramethylpiperidine-N-oxide
[0036] At 0°C, add 300ml of dichloromethane, 60ml of pyridine, 30g of 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxide into a 1L three-necked flask, and then dropwise add 66g of p-toluenesulfonate A solution of acid chloride dissolved in 200ml of dichloromethane was reacted for 180 minutes. Add dropwise to this solution again the 200ml dichloromethane solution that contains 52.4g sodium cyclohexoxide, stir and react for 120 minutes. After the reaction is complete, wash the reaction solution with water three times, dry the organic layer with anhydrous sodium sulfate, evaporate dichloromethane to dryness, add 100ml of n-hexane, freeze and crystallize, and filter to obtain 4-isopropoxy-2,2,6,6- 32 g of red crystals of tetramethylpiperidine-N-oxide. 1 H NMR (CDCl 3 , 300MHz, δ) 1.39-1.46 (m, 8H) 1.71 (s, 12H) 1.73 (m, 2H) 1.95-2.20 (m, 4H) 2.79 (m, 1H) 2.83 (m, 1H); Elemental analysis: C , 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com