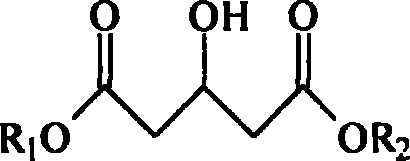
Process for preparing 3-hydroxyglutarate compound
A technology of hydroxyglutarate and oxoglutarate, which is applied in the field of preparation of ester compounds, can solve the problems of high price, hydrogen cannot be used in production, and increased difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0012] The preparation method provided by the invention is carried out in a reactor. Under lower pressure, the reaction pressure is 0.1-3Mpa, especially preferably 0.5-1Mpa.
[0013] The catalyst suitable for this method is highly active nickel or palladium-carbon catalyst, palladium-carbon or high-activity nickel containing 5%-20% of palladium is more effective, preferably high-activity nickel catalysis.
[0014] The ester solvents suitable for this method can be selected from one or more mixed solvents such as esters, saturated hydrocarbons or saturated halogenated hydrocarbons, alcohols, etc., wherein lower esters such as ethyl acetate, methyl acetate, ethyl formate, methyl formate and Lower alcohols such as methanol and ethanol are more effective, and the preferred solvent is lower aliphatic esters, especially ethyl acetate.
[0015] The reaction temperature suitable for this process is 20-120°C, particularly preferably 60-90°C.
[0016] The reaction time suitable for th...
Embodiment 1
[0024] 400kg of ethyl acetate, 150kg of diethyl acetone dicarboxylate and 25kg of highly active nickel treated with sodium hydroxide-ethanol were put into a high-pressure reactor under the protection of nitrogen, and the hydrogen pressure was raised to 0.4Mpa after the nitrogen was replaced by hydrogen. Close the hydrogen gas inlet valve, heat to 50°C under stirring, turn on the hydrogen gas when the pressure drops, turn off the heating, and feed hydrogen gas to the pressure of 0.8Mpa under natural cooling, calculate the amount of hydrogen gas to be fed, and turn on when the temperature naturally rises to 65°C Circulating water cools the reactor so that the temperature is controlled at 65-85°C, and the amount of hydrogen inhaled reaches the calculated amount within about 2-1 hours. The pressure in the constant temperature reactor does not drop any more, and then keeps stirring for 0.5 hours to terminate the reaction. Replace the hydrogen in the kettle with nitrogen, release the...
Embodiment 2
[0026] The above diethyl acetone dicarboxylate was changed to dimethyl acetone dicarboxylate, and the same operation method was adopted, and the result obtained was detected by GC, and dimethyl acetone dicarboxylate was 0.7%, and the weight was 150.8 kg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com