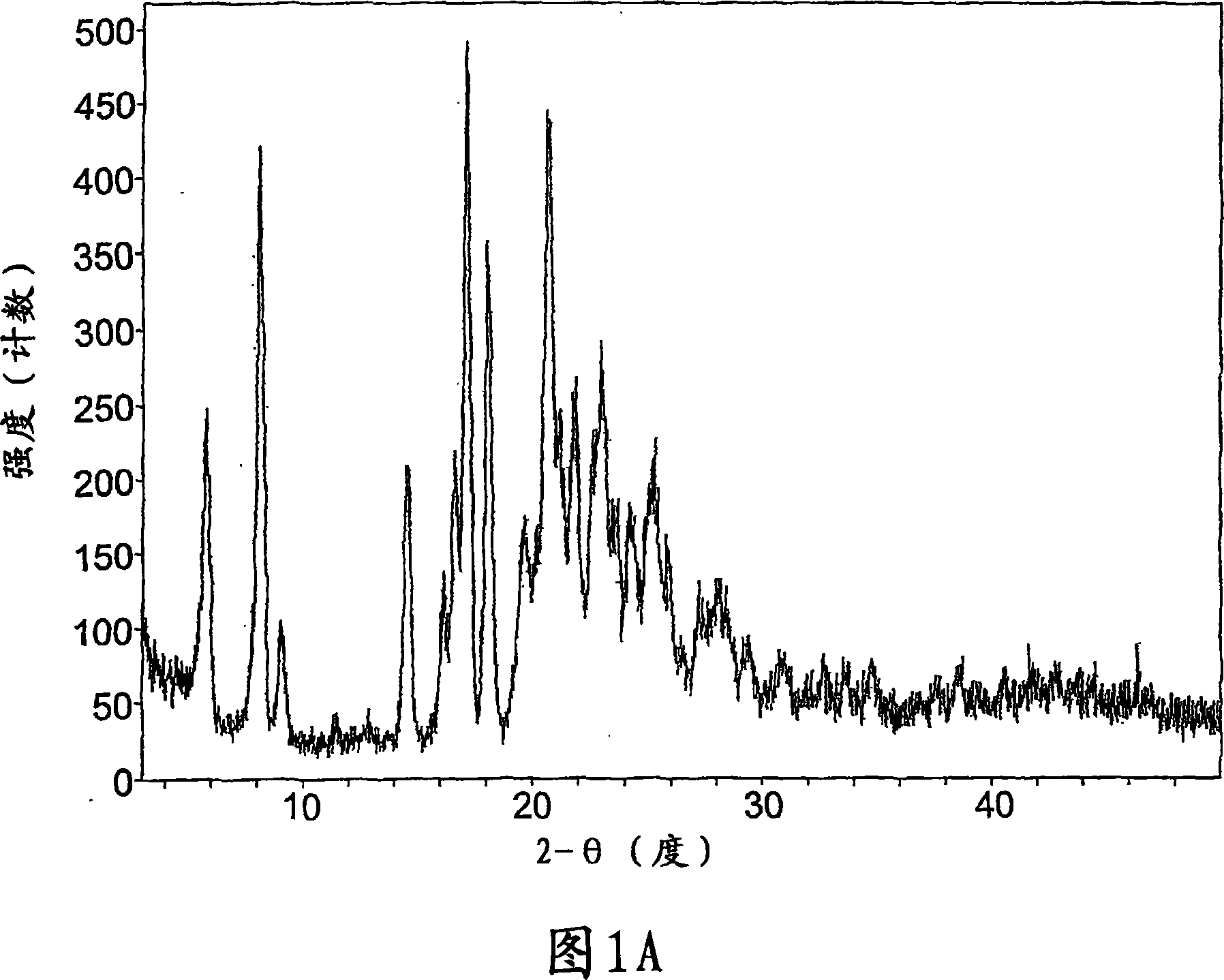
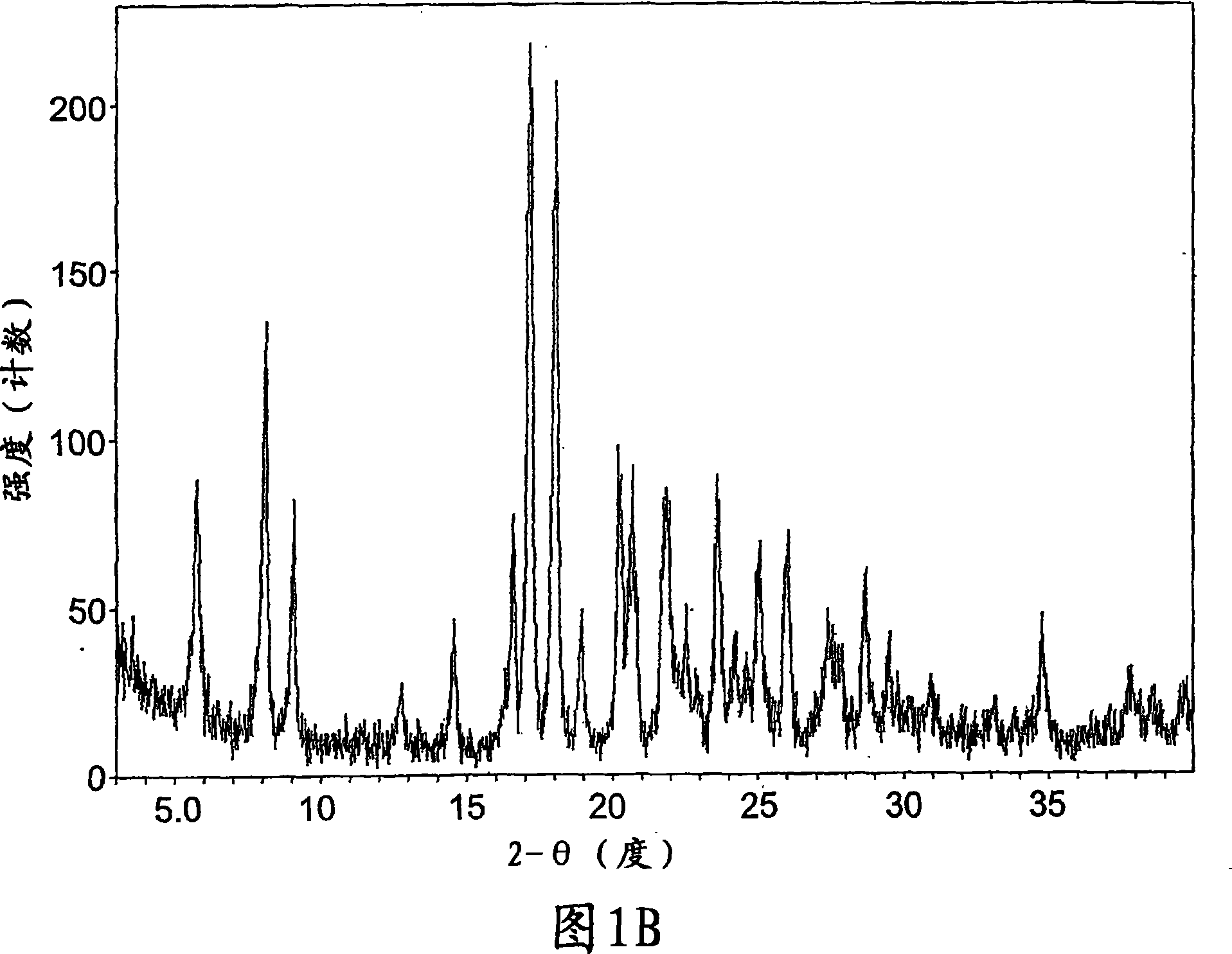
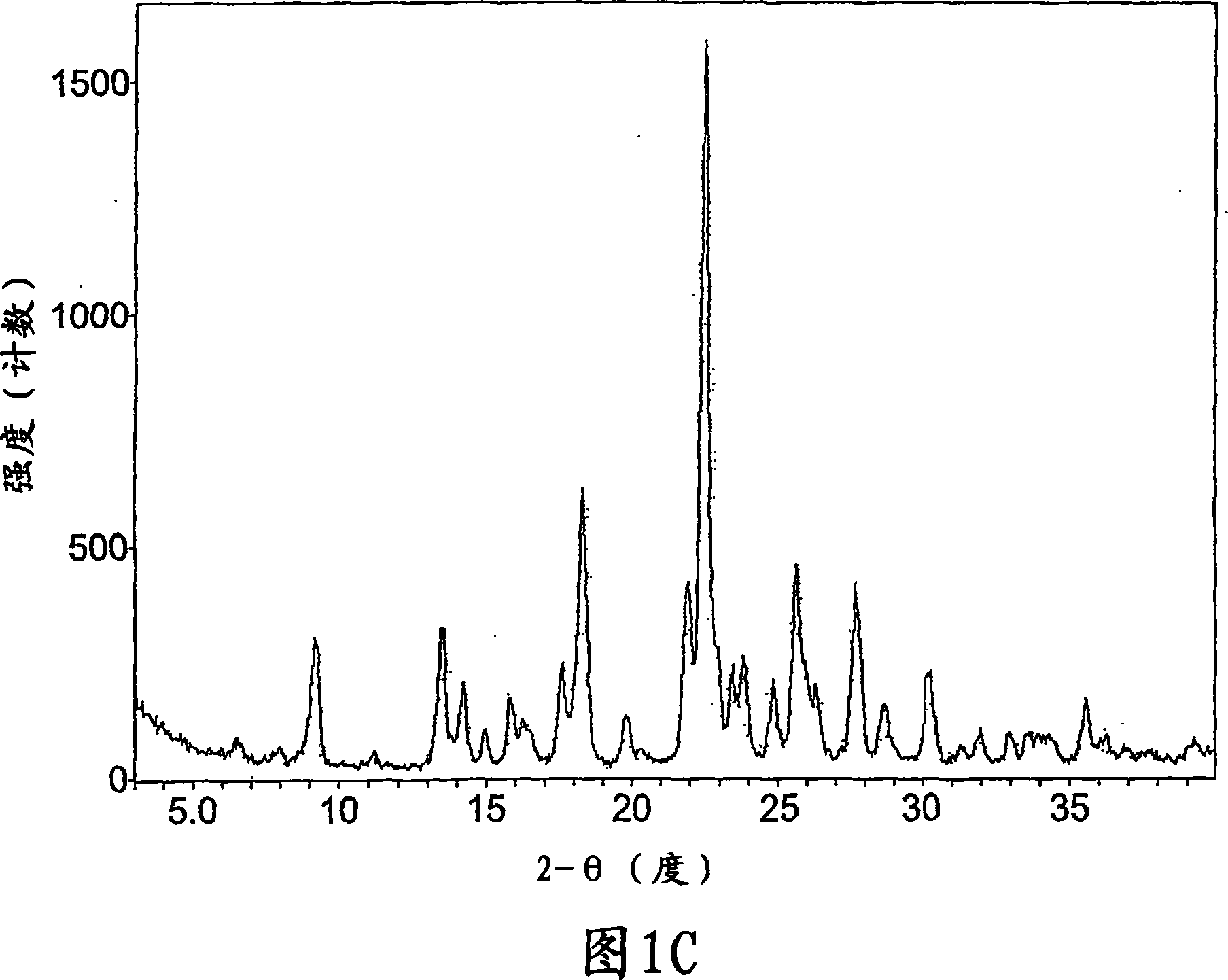
Crystalline forms of a known pyrrolidine factor Xa inhibitor
A pyrrolidinedicarboxamide and crystallization technology, applied in 1 field, can solve problems such as unpredictable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] (2R,4R)-4-Methoxy-pyrrolidine-2-carboxylic acid [2-fluoro-4-(2-oxo-pyridin-1-yl)phenyl]-amide
[0103] Step 1 Preparation of (2R, 4R)-4-methoxy-pyrrolidine-1,2-dicarboxylic acid 1-tert-butyl ester
[0104] To a nitrogen purged, 500ml 3-neck flask equipped with a mechanical stirrer and thermocouple was added 60% (w / w) NaH (8g, 200mmol) and hexane (250ml). The mixture was stirred for 1 min, after which time agitation was stopped and a solid was allowed to settle. Hexane was removed using a candle filter. THF (250ml) and CH were then added to the flask 3 I (6.51ml, 105mmol) and the resulting mixture was cooled to 0°C in an ice bath. Then, (R,R)-4-hydroxy-pyrrolidine-1,2-dicarboxylic acid 1-tert-butyl ester (22 g, 95 mmol) was added in portions while maintaining the reaction temperature at 5°C or lower. The reaction was allowed to warm to room temperature overnight. Add H to the reaction mixture 2 O (100ml), 1N HCl (100ml) and NaCl (42g). The reaction was stirred for...
Embodiment 2
[0112] From amorphous 1,2-pyrrolidinedicarboxamide, N1-(4-chlorophenyl)-N2-[2-fluoro-4-(2-oxo-1(2H)-pyridyl)phenyl]- 4-Methoxy, (2R,4R)-(9C1) Synthesis Form A.
[0113] 1.8g of amorphous 1,2-pyrrolidinedicarboxamide, N1-(4-chlorophenyl)-N2-[2-fluoro-4-(2-oxo-1(2H)-pyridyl)phenyl ]-4-Methoxy-, (2R,4R)-(9C1 ) (prepared as described by Bigge et al. in Example 150 of US2003 / 016272787) was slurried in 100 ml of water at room temperature for three days. The solid was filtered off, washed with 50 ml of water and dried under vacuum overnight to give 1.44 g of 1,2-pyrrolidinedicarboxamide, N1-(4-chlorophenyl)-N2-[2-fluoro-4-(2-oxo Substitute-1(2H)-pyridyl)phenyl]-4-methoxy-, (2R,4R)-(9C1). PXRD and DSC confirmed that the crystalline form was Form A.
Embodiment 3
[0115] Synthesis of Form A from Form B
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com