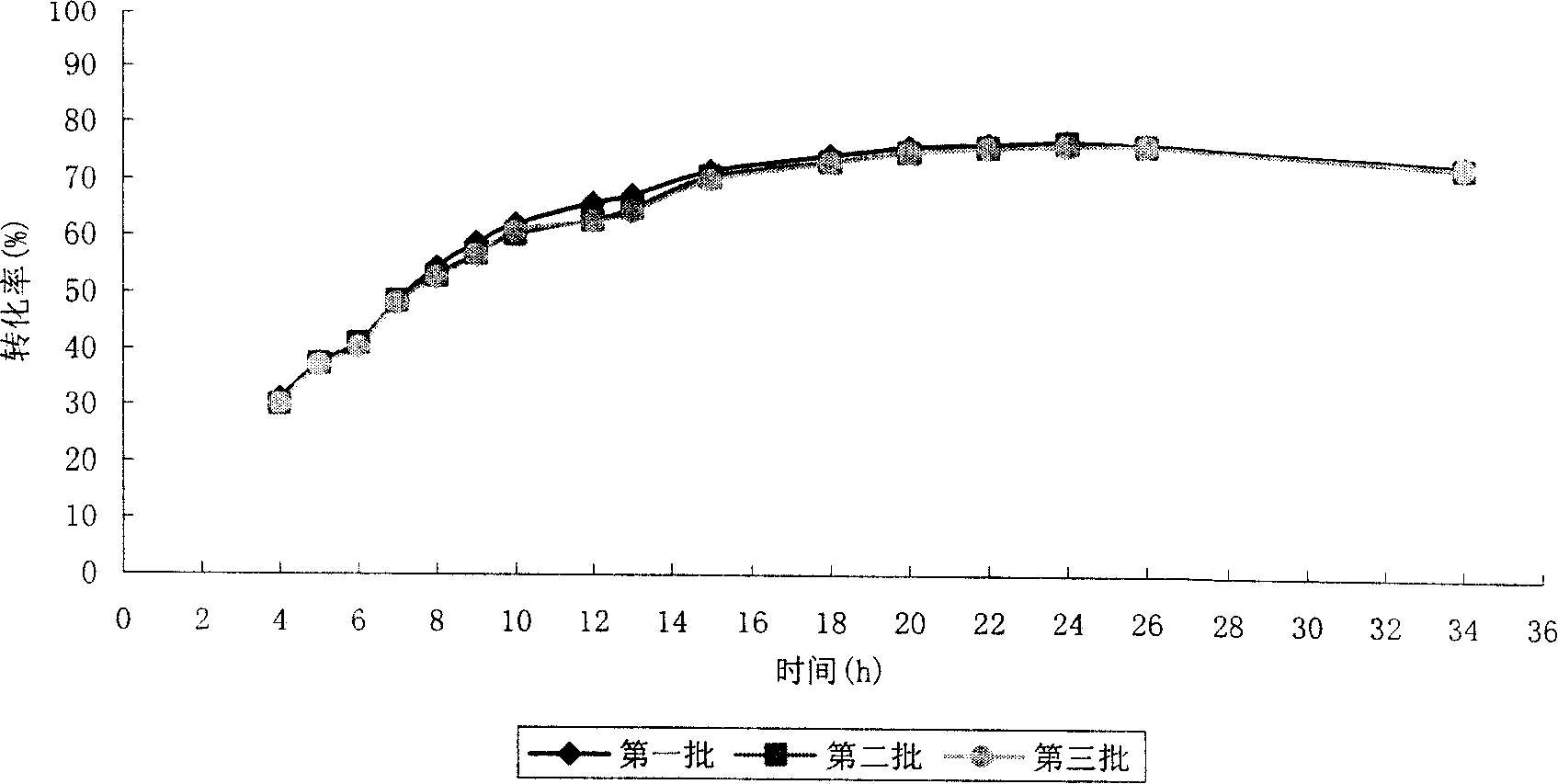
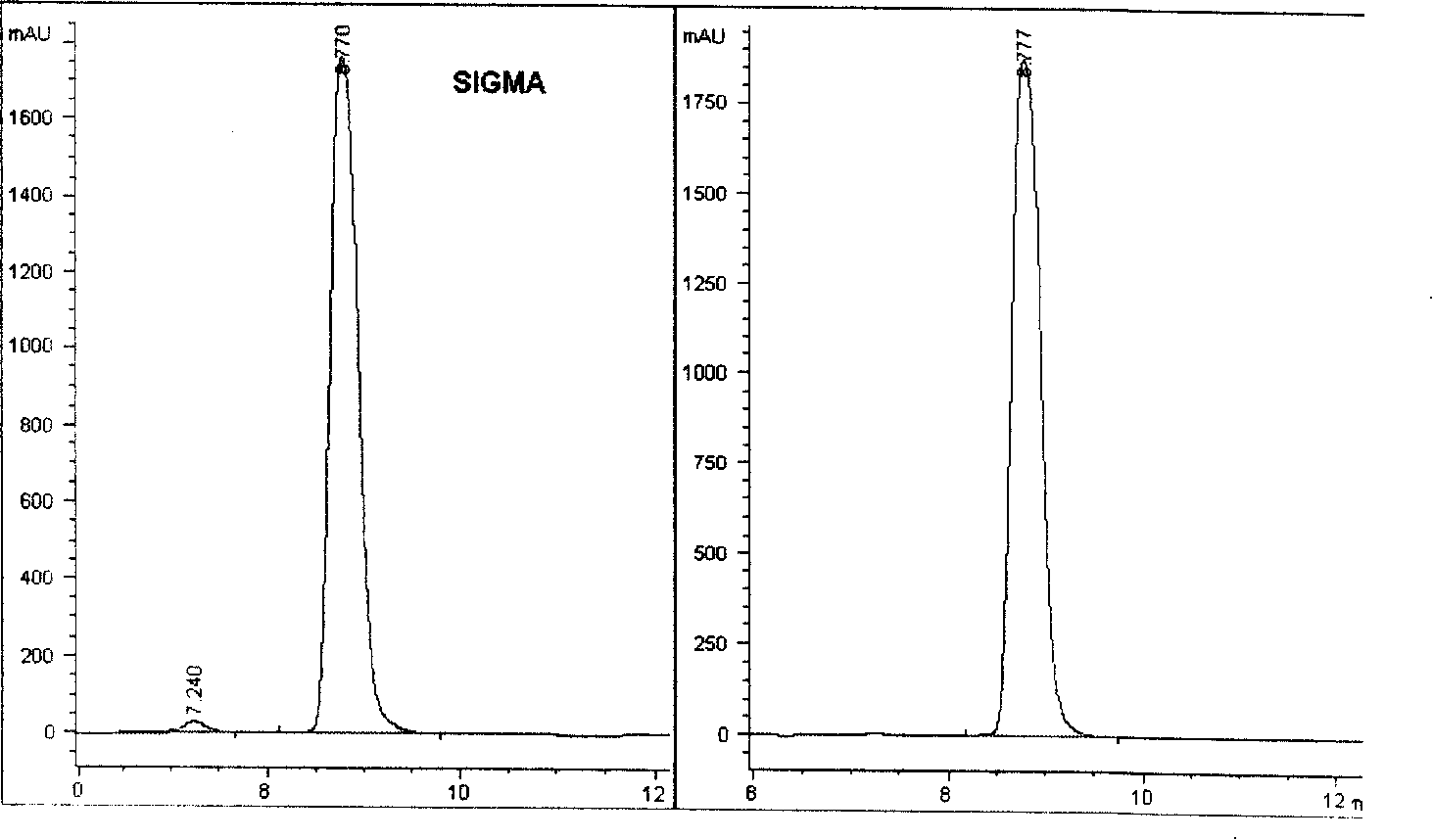
Preparation for N-acetylneuraminic acid by immobilization double-enzyme method
A technology of acetylneuraminic acid and neuraminic acid aldolase, which is applied in the field of preparing N-acetylneuraminic acid, and can solve the problems of long reaction time and the inability to reuse enzymes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Acquisition of N-acetylglucosamine-2-epimerase (E.C.5.1.3.8) gene from porcine kidney and construction of recombinant plasmid pRYepi
[0109] with the following primers
[0110] 1: 5'catatggagaaggagcgcgaa 3' (Seq.ID.No.1)
[0111] 2: 5'gaattctaggcgaggcggctcagca 3' (Seq.ID.No.2)
[0112] As primers, pig kidney total RNA was used as a template for reverse transcription PCR amplification, and after PCR, the reference molecular clone was subjected to 0.9% agarose gel electrophoresis. After electrophoresis, the 1.2Kb size fragment was recovered using the gel recovery kit.
[0113] The obtained DNA fragment was subcloned into the pKS vector, digested with Nde I and EcoR I, recovered after gel electrophoresis, and the vector pET28(b) was ligated with the former by T4 ligase overnight at 16°C after the same treatment. The overnight ligated DNA was transformed into E.coli DH5α competent cells. Recombinants were selected on LB plates containing kanamycin. The recombinant DNA...
Embodiment 2
[0115] Acquisition of N-acetylneuraminic acid aldolase (E.C.4.1.3.3) gene from total DNA of E.coli TG1 and construction of recombinant plasmid pNA1
[0116] with the following primers
[0117] 1: 5'gacgctaccatggcaacgaatttacgt 3' (Seq.ID.No.3)
[0118] 2: 5'gatccagtcgactcgcccgcgctcttg 3' (Seq.ID.No.4)
[0119] As a primer, the total DNA of E.coli TG1 was used as a template for PCR amplification. After PCR, the recovered 0.9kb fragment was digested with Nco I and HindIII, cloned into pUC118 plasmid, named pNA, and transformed into E.coli TG1. coli DH5α competent cells.
[0120] After that, the following primers
[0121] 1: 5'ggaattccatatggcaacgaatttacgtggcg 3' (Seq.ID.No.5)
[0122] 2: 5'cgggatcctcacccgcgctcttgcatc 3' (Seq.ID.No.6)
[0123] As a primer, use the plasmid pNA as a template to carry out PCR amplification. After the PCR, the recovered 0.9kb fragment is digested with Nde I and BamH I, cloned into the pET28b vector plasmid, and transformed into E.coli DH5α compete...
Embodiment 3
[0125] Fermentation of engineering bacteria BL21(DE3) / pRYepi and preparation of immobilized N-acetylglucosamine-2-epimerase
[0126] Use 3L TB culture medium (to prepare broth for every increase in concentration, add in 900ml deionized water: 12g of peptone for bacterial culture; 24g of yeast extract for bacterial culture; 4ml of glycerol. Autoclave for 20 minutes, then cool the solution to 60 ℃ or below 60 ℃, then add 100ml and sterilize to get 0.17mol / LKH 2 PO 4 , 0.72mol / L K 2 HPO 4 Solution) ferment the engineered bacteria BL21(DE3) / pRYepi in a 5L fermenter, culture it at 37°C for about 1 hour, add lactose with a final concentration of 1% to induce, and lower the temperature to 22°C at the same time, add a final concentration of 0.5 % lactose induction, ferment for about 26 hours, close the tank, centrifuge at 8000rpm for 10 minutes to collect the bacteria.
[0127] Weigh 30g of the BL21(DE3) / pRYepi fermented for 26 hours, add 45ml of 0.5M pH7.5 sodium phosphate buffer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com