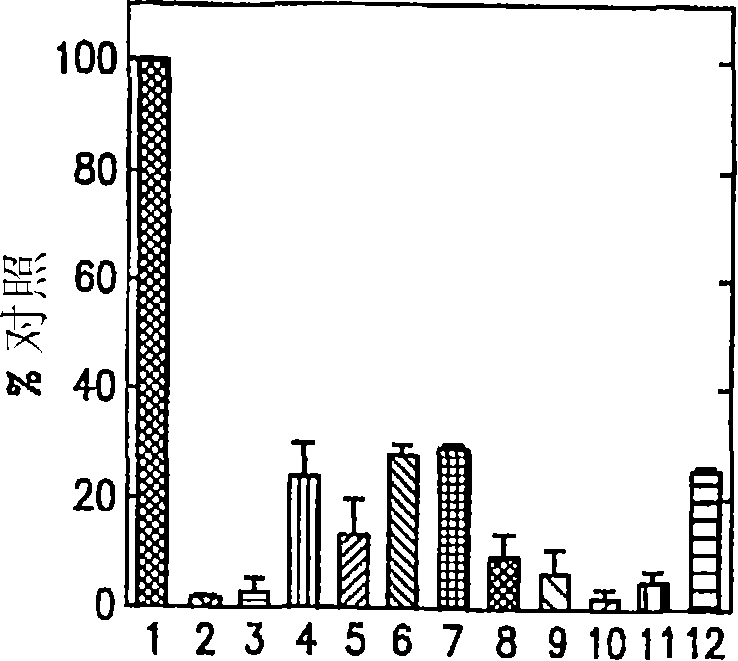
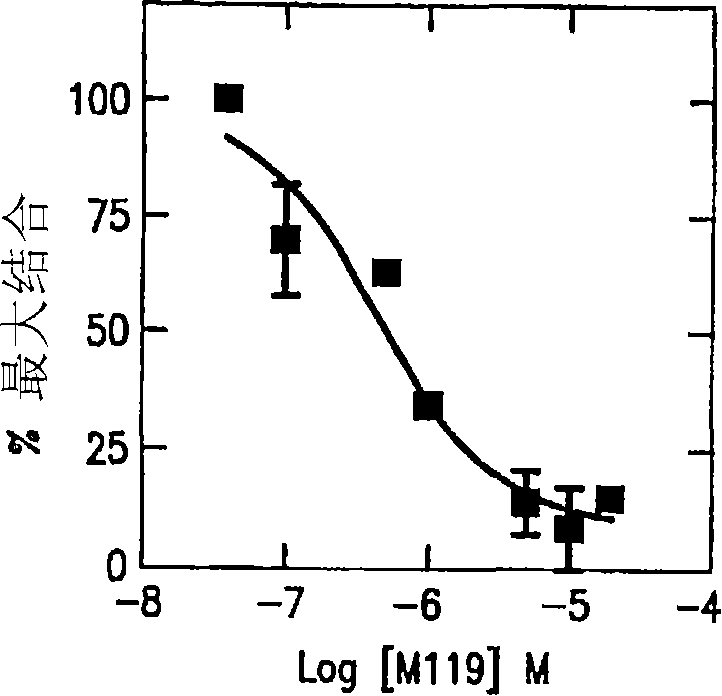
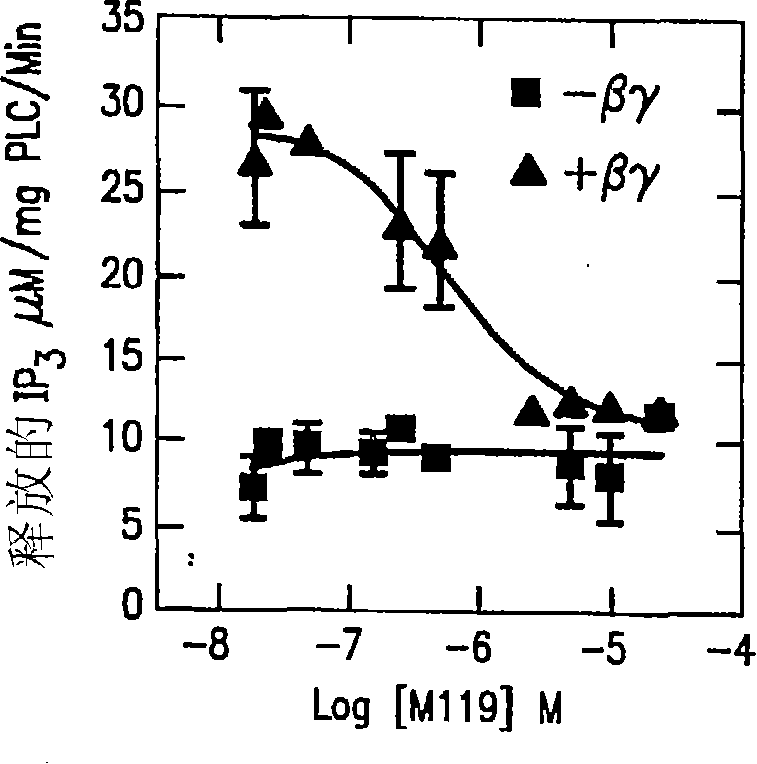
Compositions and methods for inhibiting G protein signaling
A G protein and protein technology, applied in the field of compositions and methods for inhibiting G protein signal transduction, can solve problems such as inability to promote disaggregation of heterotrimers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Example 1: Materials
[0113] Peptides were purchased from Alpha Diagnostic International (San Antonio, TX) or - Genosys (St. Louis, MO), HPLC purified to >90% and molecular weight determined by mass spectrometry. Ni-NTA agarose was purchased from (Valencia, CA). Streptavidin-coated polystyrene beads were purchased from Spherotec (Libertyville, IL). HRP-conjugated anti-M13 antibody was purchased from Amersham Biosciences (Piscataway, NJ). HRP-conjugated neutravidin was purchased from Pierce (Rockford, IL). Unless otherwise specified, all molecular biology reagents were purchased from INVITROGEN TM (Carlsbad, CA).
Embodiment 2
[0114] Example 2: Gβ 1 gamma 2 and SIGK peptide expression and purification
[0115] wild-type bovine Gβ 1 cDNA and N-terminal (His) 6 -labeled bovine Gγ 2 The cDNA of the baculovirus was used to produce the corresponding protein. with high titer of Gβ 1 and Gγ 2 Baculovirus infection of High-5 cells (INVITROGEN TM , Carlsbad, CA; 2×10 6 cells / mL). Gβ was purified using a modification of the standard method (Kozaza and Gilman (1995) J. Biol. Chem. 270:1734-41) 1 gamma 2 . All steps were done at 4 °C. The cells were harvested by centrifugation at 2600g 60 hours after infection, and then 50mL of lysis buffer (20mM HEPES, pH 8, 150mM NaCl, 5mM β-ME, 1mM EDTA, 1mL Protease Inhibitor Cocktail P-2714) to resuspend the cells. Cells were lysed by sonication and centrifuged at 2600 g to pellet membranes. Membranes were triturated to resuspend and homogenized in 100 mL lysis buffer. Add 1% of Luburol (C12E10, St. Louis, MO) and stirred to dissolve the membrane, and th...
Embodiment 3
[0117] Example 3: Crystallography
[0118] A 1.5 molar excess of peptide SIGK was added to Gβ 1 gamma 2 In, Gβ for crystallization 1 gamma 2 • The concentration of the SIGK complex was 7 mg / mL. Crystals were grown by vapor diffusion at 20°C using an equal volume (2 μL) of protein and crystallization solution (15-17% PEG 4000, 100 mM HEPES, pH 7.5, 0.01-0.05M sodium acetate, 10% glycerol). The diameter of the crystal reaches 150 μm×50 μm×20 μm within one week. Crystals were cryoprotected with 15% glycerol and frozen in liquid nitrogen.
[0119] Gβ 1 gamma 2 · SIGK’s Advanced Light Source (ALS) beamlines 8.2.1 and 8.2.2 (Berkeley, CA) and Advanced Photon Source (APS) photon beamline BM-19 (Chicago, IL) for natural crystals to filter. Data sets obtained from ALS 8.2.2 were used to determine structure. More than 100 crystals screened, diffraction limited to 7 for structure determination to 2.7 data set. The diffraction data were indexed, integrated and scaled (Table...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com