Composite preparations for curing schemic brain damage
A technology for ischemic brain injury and preparations, applied in the direction of drug combinations, medical preparations containing active ingredients, organic active ingredients, etc., can solve cardiovascular function inhibition and central nervous system dysfunction, induce gastrointestinal reactions, safety Narrow scope and other issues, to achieve the effect of improving curative effect, improving neurobehavioral disorders, and reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: A combined preparation for treating ischemic brain injury
[0036] 1. Animals and Grouping
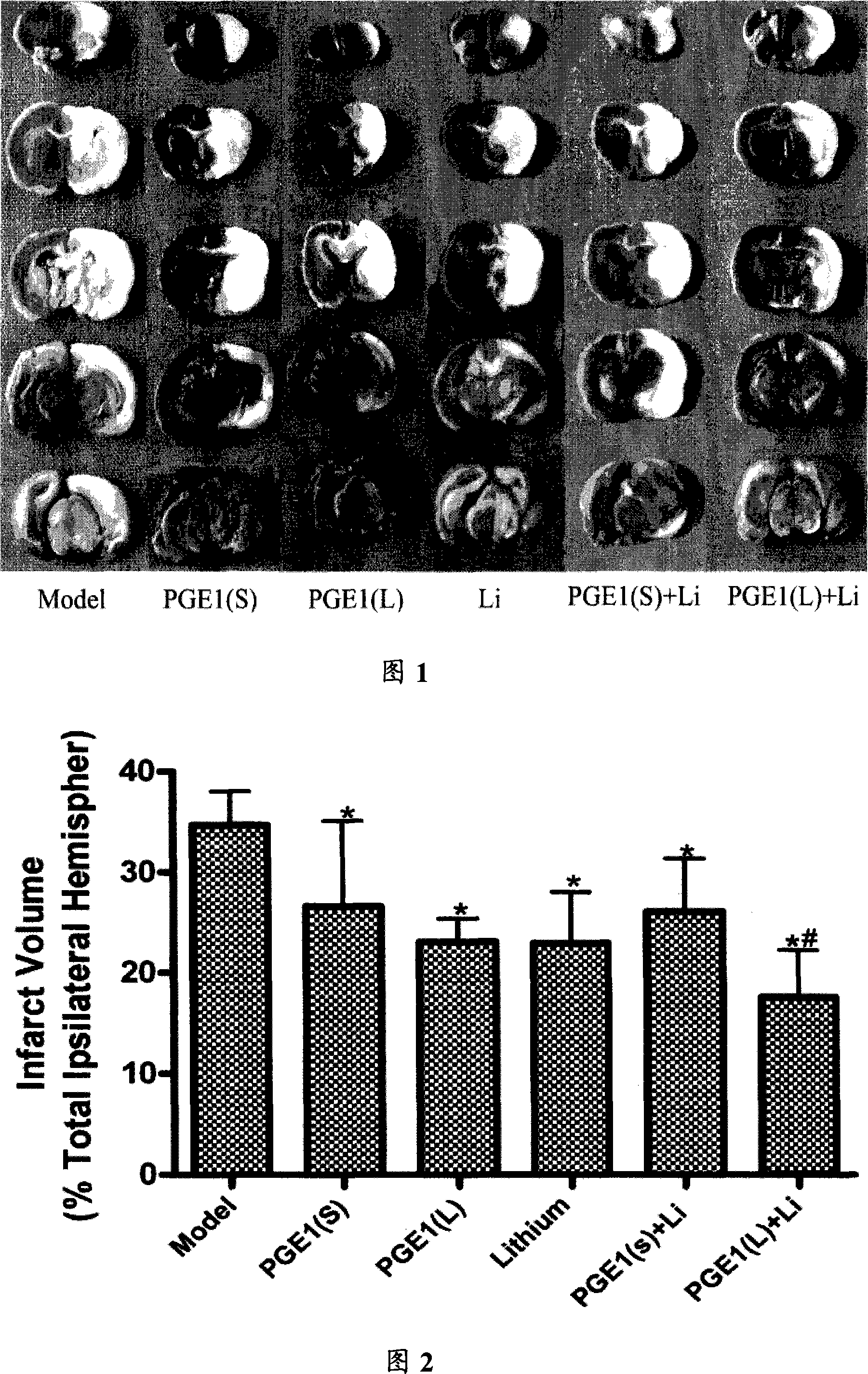
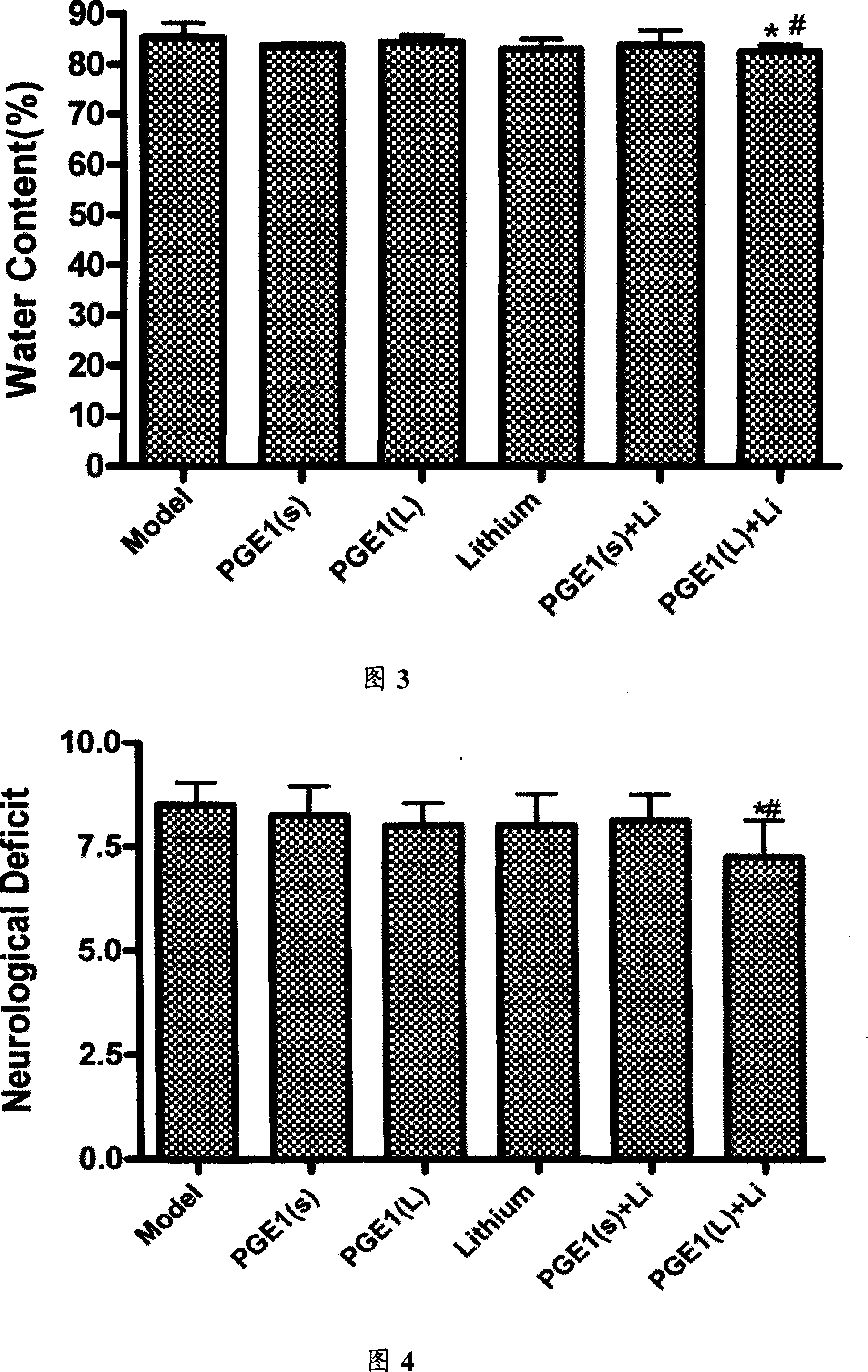
[0037] Healthy SD rats, weighing 280-310g, were divided into 7 groups, 6 rats in each group, divided into: sham operation group, model group, PGE1(L), PGE1(S), Li, PGE1(L)+Li and PGE1 (S)+Li total 7 groups. PGE1(S) group was given 22.6nmol / kg PGE1 intravenous infusion immediately after ischemia; PGE1(L) group was given 45.2nmol / kg PGE1 intravenous infusion immediately after ischemia, the speed was 1ml / h. The Li group was given subcutaneous injection of 0.5mmol / kg lithium chloride, divided into two administrations, once 24 hours before ischemia, and once half an hour before ischemia, and the PGE1(S)+Li group was given 22.6nmol / kg PGE1 intravenous infusion and 0.5mmol / kg lithium chloride subcutaneous injection were given twice; PGE1(L)+Li group was given 45.2nmol / kg PGE1 intravenous infusion and 0.5mmol / kg lithium chloride twice hypodermic injection. The kg in mol / k...
Embodiment 2
[0062] Example 2: A combination preparation for treating ischemic brain injury
[0063] 1. Animals and Grouping
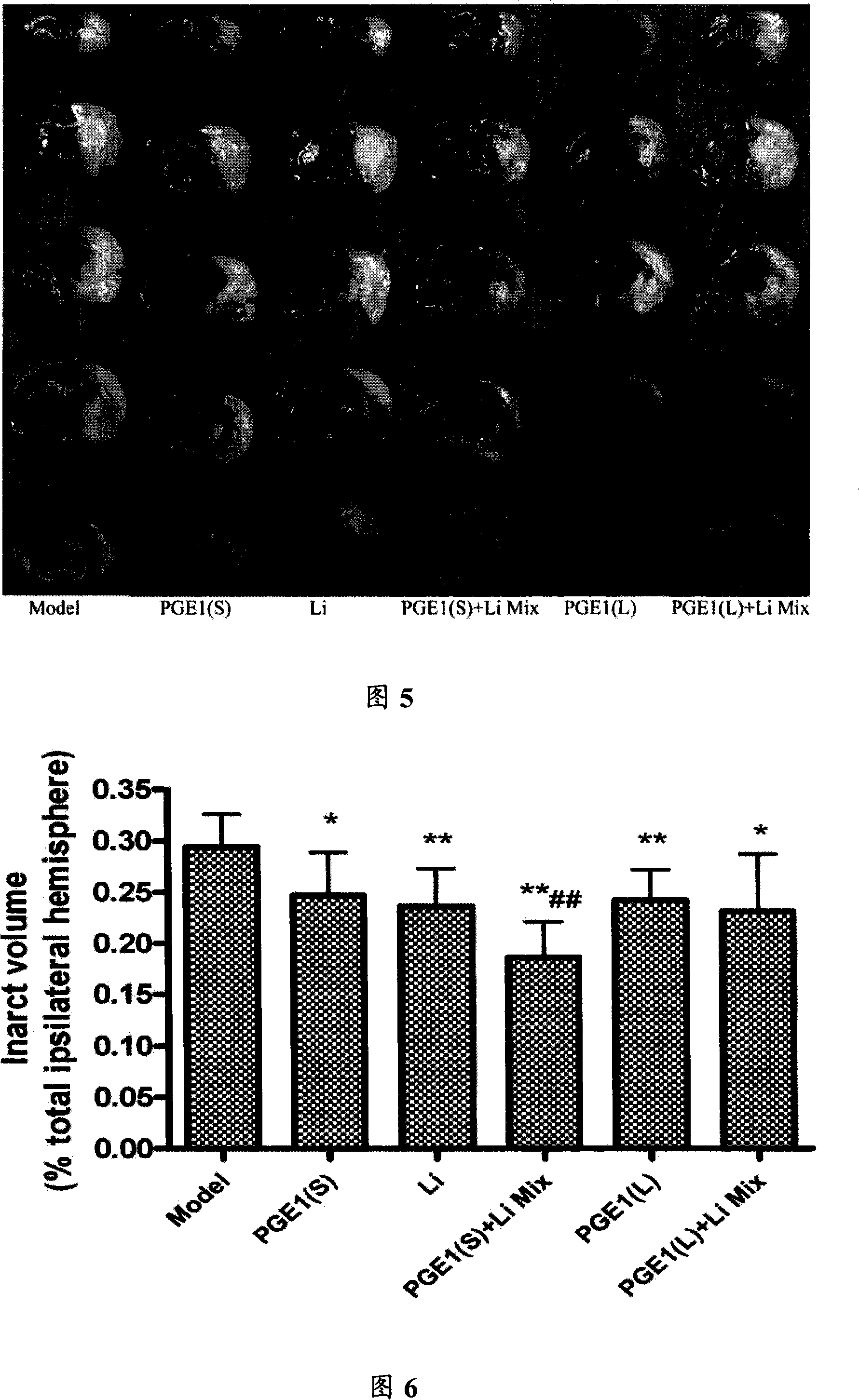
[0064] Healthy SD rats, weighing 280-310g, were divided into 6 groups, 8 in each group, namely: model group, PGE1 (L) with PGE1 intravenous infusion 45.2nmol / kg, PGE1 (S) with PGE1 intravenous infusion 22.6nmol Lithium chloride intravenous infusion 0.5mmol / kg, PGE1(L)+Li MIX (45.2nmol / kg+0.5mmol / kg) intravenous infusion mixture and PGE1(S)+Li (22.6nmol / kg +0.5mmol / kg) intravenous infusion mixture in 6 groups. PGE1, lithium chloride and PGE1+Li mixture were administered immediately after ischemia. The mixture was intravenous infusion at a speed of 1ml / h.
[0065] The doses of the above drugs are the doses used in animal experiments. When it comes to adults, the dose standard for adults should be about one-fifth to one-seventh of the dose for rats, and the dose standard for children should be used for children.
[0066] 2. Establishment of permanent focal middle ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com