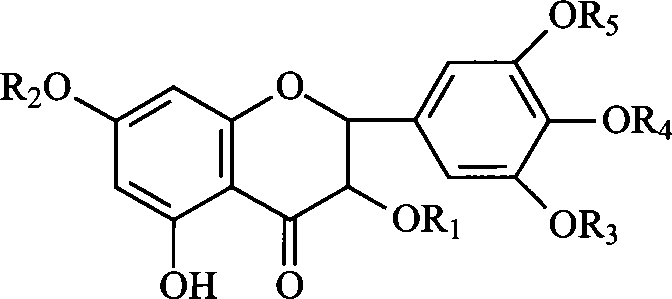
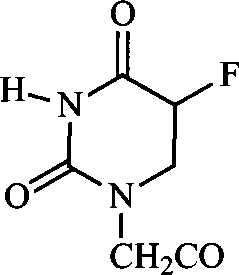
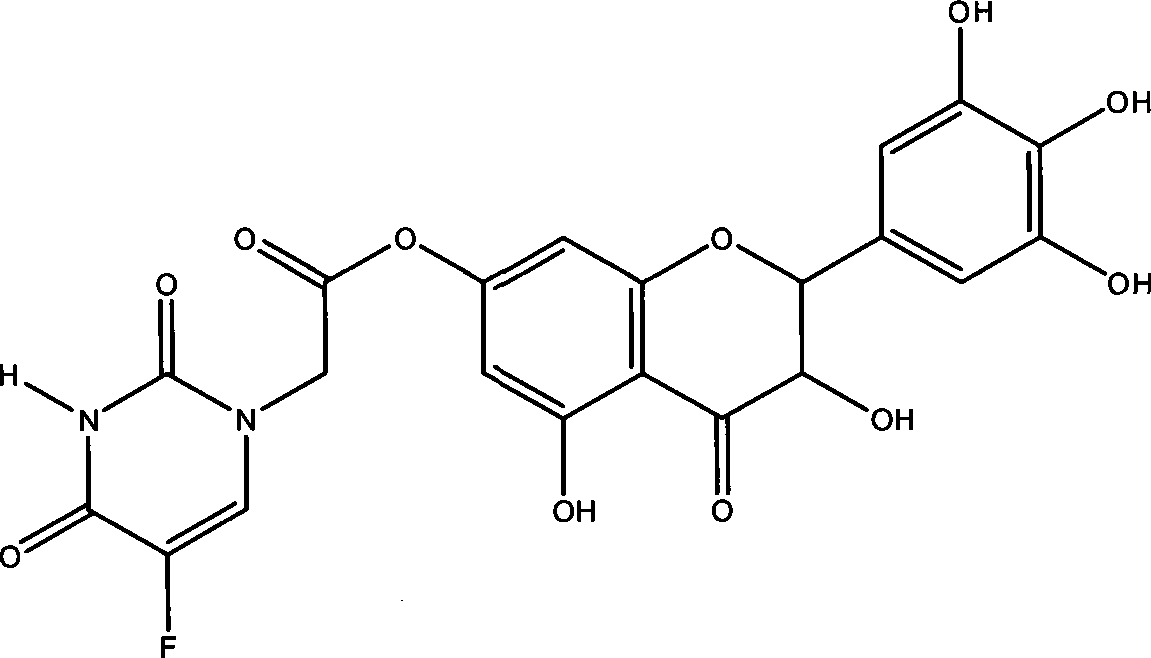
Ampelopsin derivative, synthesizing method thereof and application of the same in preparing antineoplastic medicine
A technology for the synthesis of staphylococcus, which can be used in antitumor drugs, drug combinations, medical preparations containing active ingredients, etc., and can solve the problems of low bioavailability and low lipophilicity of 5-fluorouracil, affecting anti-tumor efficacy and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of compound 3,5,3',4',5'-pentahydroxy-7-(5-fluorouracil-1-acetic acid)-2,3-dihydroflavone
[0036] 1 Experimental instruments and materials
[0037] 1.1 Instruments and equipment
[0038] Rotary evaporator: RE-52AA, Shanghai Yarong Biochemical Instrument Factory; circulating water multi-purpose vacuum pump: SHB-III, Zhengzhou Great Wall Technology Industry and Trade Co., Ltd.; collector type constant temperature heating magnetic stirrer: DF-101S, Yingyu, Gongyi City Yuhua Instrument Factory; Vacuum Drying Oven: 876-1, Shanghai Pudong Dongfeng Scientific Instrument Co., Ltd.; Electrothermal Constant Temperature Blast Drying Oven: 101-1, Shanghai Yuejin Medical Instrument Factory; pH Meter: pHS-25, Shanghai Leici Instrument Factory; constant temperature magnetic stirrer: JB-2 type, Shanghai Leici Instrument Factory.
[0039] High performance liquid chromatography: LC-10AT VP, Shimadzu, Japan; UV-visible spectrophotometer: UV4802, Unico Instrument...
Embodiment 2
[0072] Embodiment 2 Antitumor pharmacological experiments of the compound of Example 1
[0073] 1. Experimental Instruments and Materials
[0074] 1.1 Instruments and equipment
[0075] CO 2 Incubator: American Precision Company; Inverted microscope: Chongqing Optical Instrument Factory; Microplate reader: American Bio-TEK Company; Desktop low-speed centrifuge: Type 0412-1, Shanghai Medical Instrument Co., Ltd.; 96-well cell culture plate: American CORNING Company ;Cell culture flask: American CORNING company
[0076] 1.2 Drugs and reagents
[0077] RPMI-1640 medium, newborn bovine serum: GIBCO Company; green chain double antibody: Shanghai Sangong; brominated thiazolyl blue tetrazolium (MTT): SIGMA Company;
[0078] Cell line: K562 sensitive strain, Sun Yat-sen University Cancer Hospital Experimental Center
[0079] 2 Experimental methods
[0080] 2.1 Liquid preparation
Embodiment 1
[0081] The compound of Example 1 was directly dissolved in serum-free culture medium to prepare the concentration required for the experiment, and sterilized by filtration with a 0.22 μm microporous membrane, ready to use.
[0082] 2.2 Cell Culture
[0083] Human leukemia K562 cells contained 10% inactivated newborn bovine serum by volume, 1×10 5 U·L -1 penicillin and 100mg·L -1 Cultured in RPMI-1640 medium of streptomycin, placed at 37°C, CO 2 Cultured in an incubator with a content of 5%. After 2-3 days, replace the medium by centrifugation or subculture, and take the cells in the logarithmic growth phase for experiments.
[0084] 2.3 Determination of tumor cell growth inhibition rate by MTT method
[0085] Dilute the K562 cells in the logarithmic growth phase with fresh culture medium, count them, and inoculate them in 96-well culture plates, so that each well has 5×10 4 about a cell. Dissolve the compound of Example 1 with serum-free culture solution, and configure 3....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com