ChIFNGR1 gene and its coding protein and application
A technology of protein and gene, applied in the direction of peptide/protein composition, application, genetic engineering, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1. Obtaining of Chicken IFNγ Receptor Alpha Chain (chIFNGR1)
[0020] 1. The cDNA sequence of chicken chIFNGR1 was obtained by rapid amplification of cDNA ends (RACE) method
[0021] The total RNA of chicken peripheral blood lymphocytes was extracted, and the total RNA was used with 100 U MMLV-RT (promega company product), 100 ng of upstream and downstream primers 5'-AACATATGCGGCCGCATTATGGCCGGG-3' and 5'-GATCTTCCACGCGTCGAC(T)30MN-3'(M =A, G, C; N=A, G, C, T) Reverse transcription was performed at 42°C. The obtained cDNA was subjected to 3'RACE, and RACE primers were designed according to the conserved region of the red jungle fowl IFNGR1 computer predicted sequence XM_419722 registered on GenBank, and the primers for the first round of PCR were specific upstream primers GSP1 (5'-CAGAAGCAAAAGGCATTTGTGCCGTC-3') and Universal downstream primer UP (5'-GATCTTCCACGCGTCGACT-3'), primers for the second round of PCR are nested upstream primers GSP2 (5'-TGTGAGTGTCCCATTGA...
Embodiment 2
[0027] Embodiment 2, expression of chicken IFNGR1 extracellular domain (EC) recombinant protein
[0028] 1. Construction of chicken IFNGR1EC recombinant expression vector
[0029] The extracellular domain of chicken IFNGR1 was amplified by conventional PCR, and the primers used were chIFNGR1EC-F (5'-TAGGATCCGAGCGTCTTCCCGCAGT-3) and chIFNGR1EC-R (5'-GCTCTAGATCAAGCCTGCGTGATAGGAACC-3'), and the PCR reaction conditions were: 94°C for 5 minutes, 94°C for 1 minute, 60°C for 1 minute, 72°C for 1 minute, 30 cycles; 72°C for 10 minutes. After the reaction, the amplified PCR product was double-digested with BamHI and Xba I, and inserted into the pMAL-p2x (product of New England Biolabs) vector, and the ligated product was transformed into Escherichia coli competent cell DH5α and sequenced. A recombinant plasmid containing IFNGR1EC was obtained and named pMAL-p2x / IFNGR1EC.
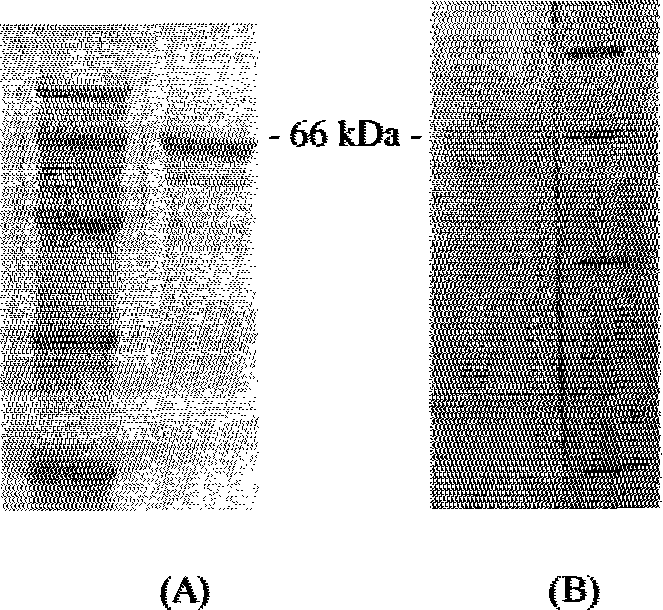
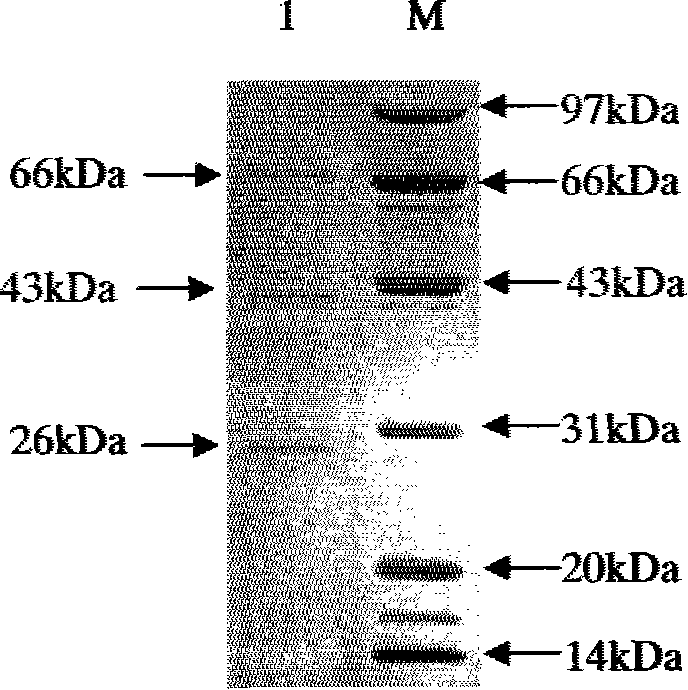
[0030] 2. Transform Escherichia coli TB1 competent cells with the recombinant plasmid in step 1, and shake the b...
Embodiment 3
[0031] Embodiment 3, the determination of antiviral activity of chicken IFNGR1 ectodomain (EC) recombinant protein
[0032] After the concentration of the purified rIFNGR1EC protein was determined, the lesion-inhibiting effect test was carried out. The rIFNGR1EC tagged with maltose binding protein (MBP) was serially diluted (1:4), inoculated on the monolayer of chicken CEF cells, and after culturing for 24 hours, 100 TCID was added to each well 50 Equine vesicular stomatitis virus) (VSV), when the virus control wells were completely diseased, the antiviral activity of rIFNGR1EC was calculated using the calculation method of interferon titer. rIFNGR1EC has high antiviral activity and can effectively inhibit the destructive effect of VSV on fibroblasts. The antiviral activity of rIFNGR1EC was 4.58×10 3 U / mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com