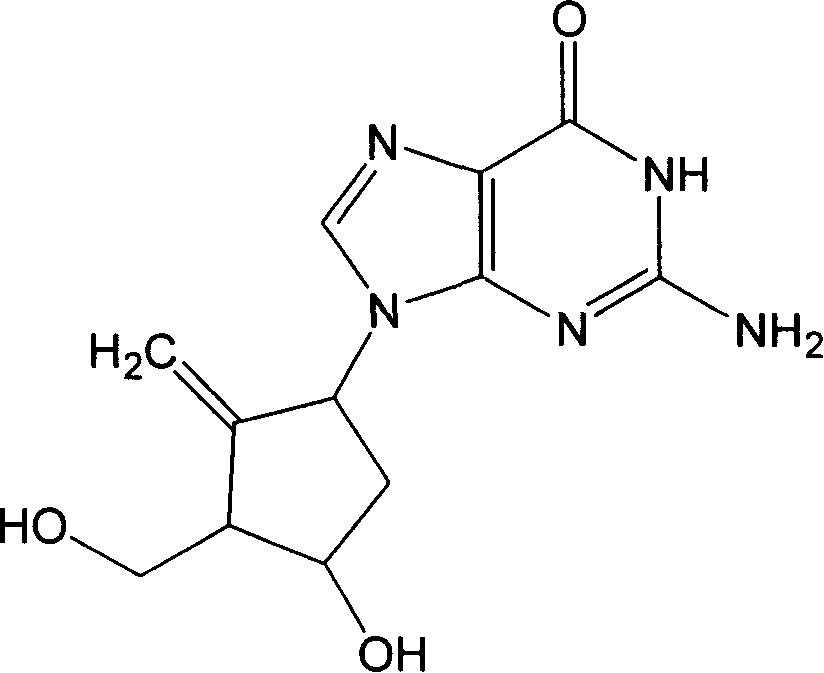
Entecavir kalium, preparation method and application thereof
A technology of Entecavir and Cavir Potassium, which is applied in the field of potassium salt compound of Entecavir and its preparation, can solve the problems of improvement, low solubility of Entecavir in water, unfavorable preparation of oral preparations and bioavailability, and achieve good therapeutic effect and good water solubility performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
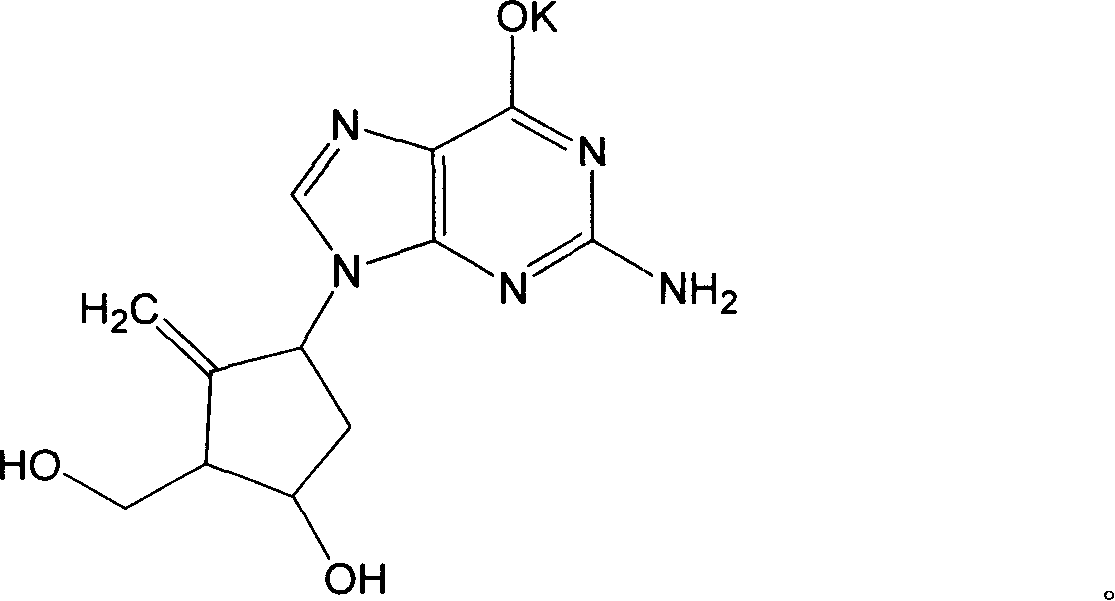
[0027] Embodiment 1. Preparation of Entecavir Potassium
[0028] Take 5 grams of Entecavir, add potassium hydroxide aqueous solution to dissolve, adjust the pH value to 11, add about 65ml of ethanol while stirring, continue stirring for 10 minutes, let stand for 2 hours, filter with suction, and wash the filtered precipitate with ethanol for 2 to 3 times Afterwards, adopt vacuum drying method, dry at 60 ℃ for 4 hours, obtain off-white solid powder, be entecavir potassium, molecular formula C 12 h 14 N 5 o 3 K, elemental analysis result (%): C 45.76, H 4.44, N 22.27, K 12.36, theoretical value (%): C 45.70, H 4.47, N 22.21, K 12.40, elemental analysis value is consistent with theoretical value.
[0029] NMR analysis 1 H-NMR δ(ppm) (400MHZ, DMSO): 1.79-2.04 (2H, multiplet), 2.0 (1H, singlet), 2.33 (1H, multiplet), 3.24 (1H, multiplet), 3.40-3.65 (2H, multiplet), 4.37 (1H, multiplet), 4.87 (1H, doublet), 4.95 (1H, doublet), 8.68 (1H, doublet).
Embodiment 2
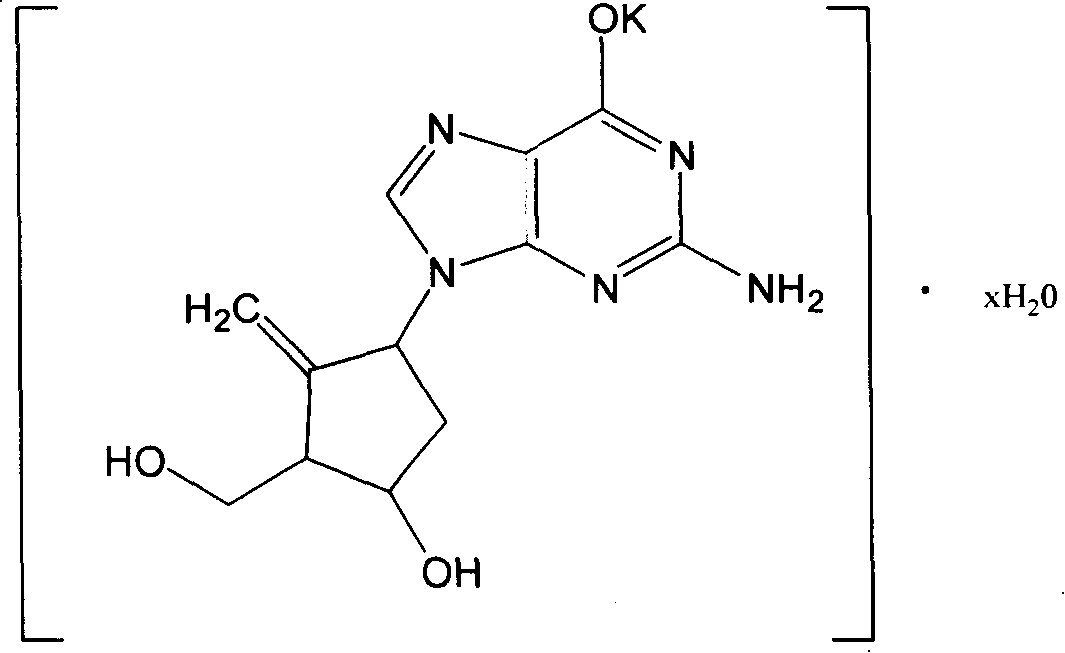
[0030] Example 2. Preparation of Entecavir Potassium Crystal Hydrate
[0031] Take 5 grams of Entecavir, add potassium hydroxide aqueous solution to dissolve, adjust the pH value to 13, add about 70ml of acetone and ethanol mixed solution (V:V=1:1) under stirring, continue stirring for 10 minutes, let stand for 2 hours, pump After filtering, the filtered precipitate was washed with ethanol for 2 to 3 times, dissolved in water, and vacuum dried at 60°C for 4 hours to obtain an off-white solid.
[0032] According to thermogravimetric and differential thermal analysis, it contains a molecule of crystal water, which is entecavir potassium monohydrate, molecular formula C 12 h 14 N 5 o 3 K·H 2 O.
[0033] Elemental analysis results (%): C 43.20, H 4.88, N 21.06, K 11.68, theoretical values (%): C 43.23, H 4.84, N 21.01, K 11.73, elemental analysis values are consistent with theoretical values.
[0034] NMR analysis H 1 -NMR δ (ppm) (400MHZ, DMSO): 1.78-2.05 (2H, multiple...
Embodiment 3
[0035] Embodiment three. the preparation of entecavir potassium tablet
[0036] Get 1.15 grams of Entecavir Potassium prepared in Example 1, add an appropriate amount of carrier auxiliary materials (such as starch, lactose, mannitol, dextrin, hydroxypropyl cellulose, povidone, magnesium stearate, etc.), wet granulation, drying Dry, compress into tablets with a tablet machine, each tablet has a specification of 0.5mg (calculated as entecavir), for oral use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com