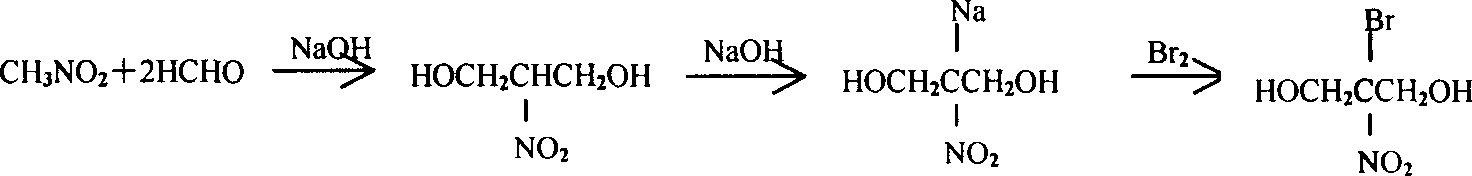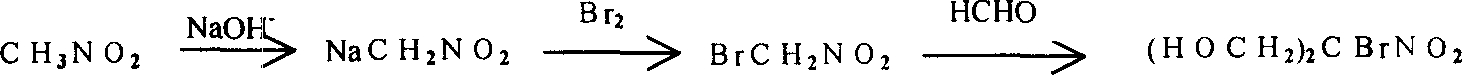Improved method for synthesizing bronopol
A synthesis method and bronopol technology are applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve the problems of high production cost, easy explosion of nitro compounds, and safety, and achieve low consumption. , the effect of avoiding the explosion of nitro compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 130.0g of 37% formaldehyde, 64.0g of methanol, and 80.0g of deionized water into a 500ml four-necked flask, cool down to below 4°C with an ice-salt bath under stirring, add 15.0g (0.15mol) of 40% sodium hydroxide solution, 48.8 g (0.80 mol) of nitromethane was added dropwise, and the condensation reaction temperature was controlled at 4-10° C. When the solution temperature dropped to 4° C., stirring was continued for 10 minutes to complete the condensation reaction. Then 65.0 g (0.65 mol) of 40% sodium hydroxide solution was added dropwise, and the neutralization reaction temperature was controlled at 4-20° C., and the neutralization reaction was completed when the temperature no longer rose. Under stirring, the reactant was further cooled to below -4°C, and then 67.2g (0.42mol) of bromine was added dropwise, and the reaction temperature was controlled at -4-0°C. When the bromine color faded, the bromination reaction was completed by 50%-55%. Then feed about 27.0 g ...
Embodiment 2
[0024] Add 48.8 g (0.80 mol) of nitromethane and 440 g of deionized water into a 500 ml four-necked flask, cool down to below 4°C with an ice-salt bath under stirring, and add 80.0 g (0.80 mol) of 40% sodium hydroxide solution dropwise, The reaction temperature is controlled at 4-20° C., and the neutralization reaction is completed when the reaction temperature no longer rises. Under stirring, it was further cooled to below -4°C, then 67.2g (0.42mol) of bromine was added dropwise, and the reaction temperature was controlled at -4-0°C. When the color of bromine faded away, the bromination reaction was 50% complete. Then feed about 27.0 g (0.38 mol) of chlorine gas to continue the bromination reaction, and the chlorination process ends when the pH value of the reaction solution becomes acidic. The reaction solution was steam-distilled to separate the organic phase to obtain 112.0 g of bromonitromethane product. According to gas chromatography analysis, the bromonitromethane prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com