Method for preparing aldehydes or ketones compounds by selective catalytic oxidation of alcohols compounds
A ketone compound, catalytic oxidation technology, applied in the oxidation preparation of carbonyl compounds, organic chemistry and other directions, can solve the problem of expensive catalyst, and achieve the effect of easy separation and recycling, low cost and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
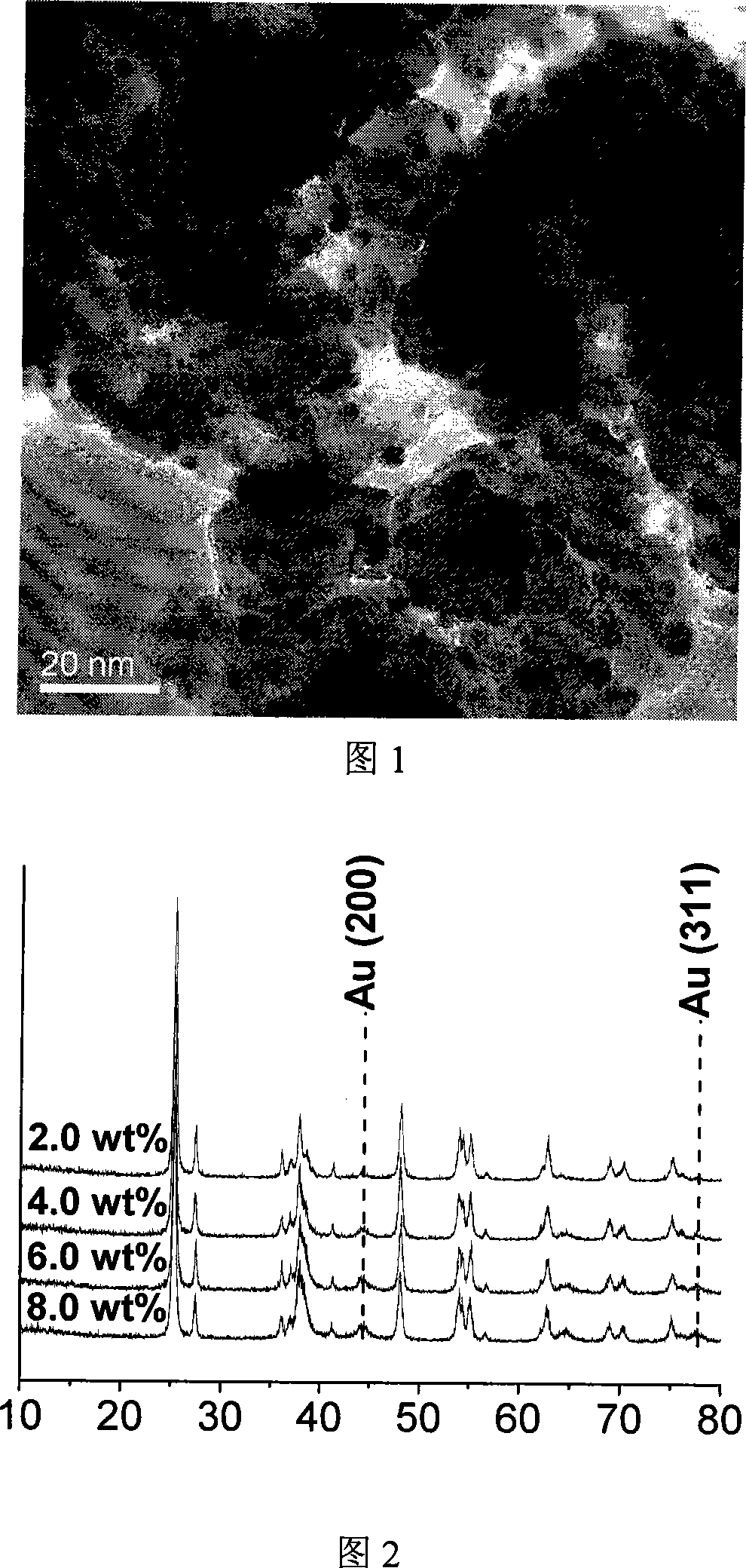
[0015] 1. Preparation of Titanium Dioxide Supported Gold Catalyst
[0016] with HAuCl 4 4H 2 O is the precursor of gold, TiO 2 (P25) was used as catalyst carrier, urea was used as precipitant, and Au / TiO was prepared by deposition-precipitation method 2 catalyst. 1g.TiO 2 respectively added to 25mL, 50mL, 75mL or 100mL solution (where HAuCl 4 : 4.2×10 -3 M, urea: 0.42M, sodium citrate: 6.9×10 -3 M). The mixture was placed in a round-bottomed flask and stirred under strong magnetic force at 80°C for 4 hours in an oil bath. Centrifuge and wash the solid matter in the above mixture to no chloride ion exists (AgNO 3 test). The separated solid was dried in a vacuum oven at 100° C. for 2 hours. Finally, it was placed in a tube furnace and reduced in a hydrogen atmosphere at 300°C for 4 hours. Preparation of Au / TiO with different loadings 2 (2.0 wt%, 4.0 wt%, 6.0 wt% or 8.0 wt%) catalyst.
[0017] 2. Oxidation of Benzyl Alcohol Catalyzed by Titanium Dioxide Supported Go...
Embodiment 2
[0022] Embodiment 2: the catalytic oxidation of benzyl alcohol
[0023] 0.1 g of 8.0wt% Au / TiO used through three cycles 2 Put the catalyst into the autoclave, add 15mL of deionized water and 29mmol of benzyl alcohol, seal the autoclave, pass through the autoclave for four times for replacement, then pressurize to 1MPa, heat the autoclave until the temperature in the autoclave is 100°C, start stirring and timing, and react The time is 8h. After the reaction, the oil phase was separated by centrifugation, and the composition of the product was analyzed by a gas chromatograph. The results are: the conversion rate of benzyl alcohol is 80%, the selectivity of benzaldehyde is 67%, the selectivity of benzoic acid is 10%, and the selectivity of benzyl benzoate is 23%.
Embodiment 3
[0024] Embodiment 3: the catalytic oxidation of benzyl alcohol
[0025] Weigh 0.1g 8.0wt% Au / TiO 2 Put the catalyst in an autoclave, add a mixed solvent of 10mL deionized water and 10mL p-xylene, 29mmol benzyl alcohol, seal the autoclave, replace it with oxygen four times, pressurize it to 1MPa, and heat it until the temperature in the autoclave is 100 °C, start stirring and timing, and the reaction time is 8h. After the reaction, the oil phase was separated by centrifugation, and the composition of the product was analyzed by a gas chromatograph. The results are: the conversion rate of benzyl alcohol is 83%, the selectivity of benzaldehyde is 93%, the selectivity of benzoic acid is 3%, and the selectivity of benzyl benzoate is 4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com