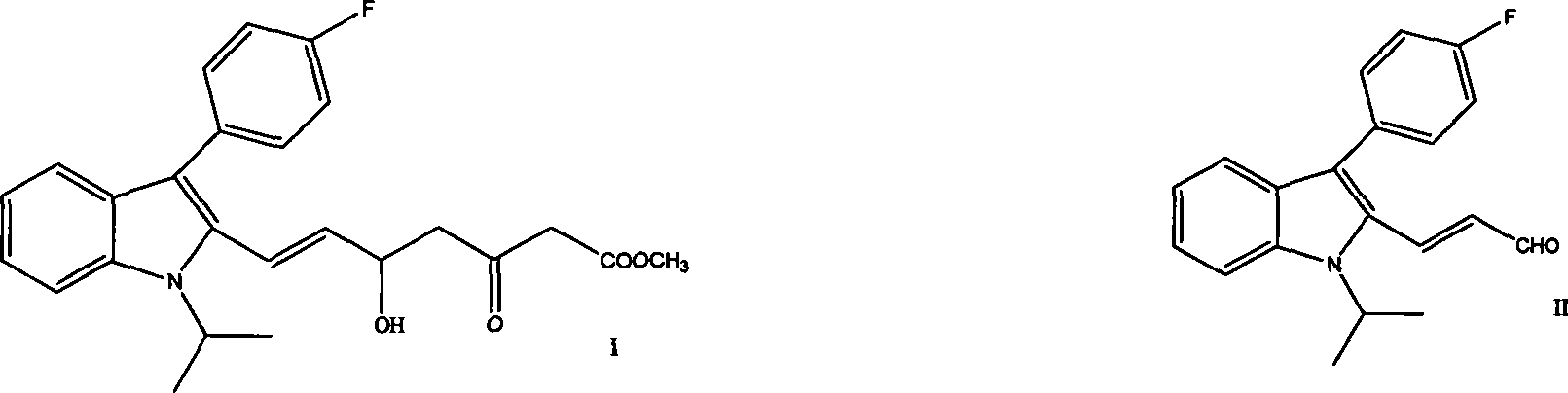Refining of fluvastatin sodium intermediate and preparation method of fluvastatin sodium
A technology of fluvastatin sodium and a refining method, which is applied in the field of compound refining, can solve the problems of no impurity removal, low purity of fluvastatin sodium, and low product purity, and achieve the goal of improving purity, improving product quality, and high purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Inside the reactor, nitrogen gas was added, anhydrous tetrahydrofuran was added, and stirred. Add 1.7 g NaH and stir for 5 minutes. The temperature was lowered to below 0°C, and 4.6 mL of methyl acetoacetate solution was slowly added dropwise. After the dropwise addition, stirring was continued below 0°C for 1 hour. Slowly add 27.5mL of n-butyllithium / n-hexane solution dropwise, stir and react at 0~5°C for half an hour, add 8g of (E)-3-[3-(4-fluorophenyl)-1-(1-methylethane base)-1H-indol-2-yl]-2-propenal / 150mL THF solution, then add a saturated ammonium chloride hydrochloric acid mixed solution, fully stir for 20 minutes, carry out liquid separation, and wash the organic layer twice with saturated saline, Dry over anhydrous sodium sulfate. After filtration, the filtrate was evaporated to dryness under reduced pressure to obtain an orange translucent oil (hereinafter referred to as "orange oil").
[0045] Dissolve 9 g of the above orange oil in 12 mL of ethyl acetate,...
Embodiment 2
[0047]Dissolve 9 g of orange oil in 54 mL of isopropanol, heat to dissolve, add 54 mL of n-heptane, stir for 2 hours, filter, and dry in vacuo at 45°C to obtain 7.5 g of yellow solid (E)-7-[3-(4-fluorobenzene yl)-1-(1-methylethyl)-1H-indol-2-yl]-5-hydroxy-3-oxo-6-heptenoic acid methyl ester. Mp: 98~100°C, HPLC: 98.7%.
Embodiment 3
[0049] Dissolve 9g of orange oil in 18mL of methanol, heat to dissolve, add 63mL of n-hexane, stir at room temperature for 2 hours, filter, and dry under vacuum at 45°C to obtain 6.7g of yellow solid (E)-7-[3-(4-fluorophenyl) - 1-(1-methylethyl)-1H-indol-2-yl]-5-hydroxy-3-oxo-6-heptenoic acid methyl ester. Mp: 99~100.5°C, HPLC: 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com