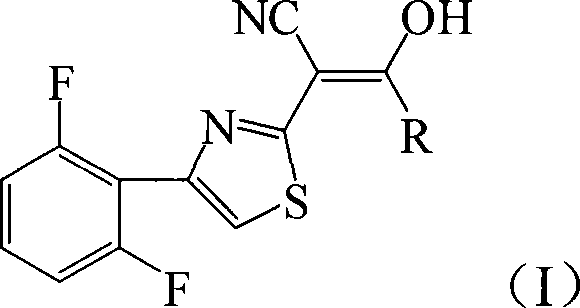
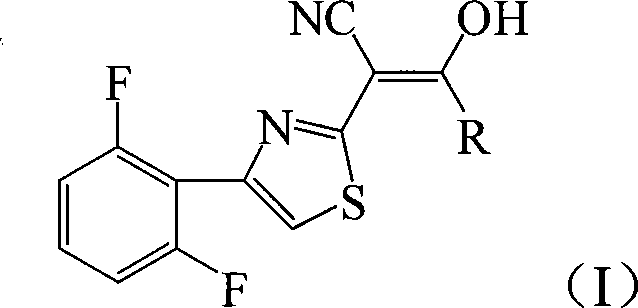
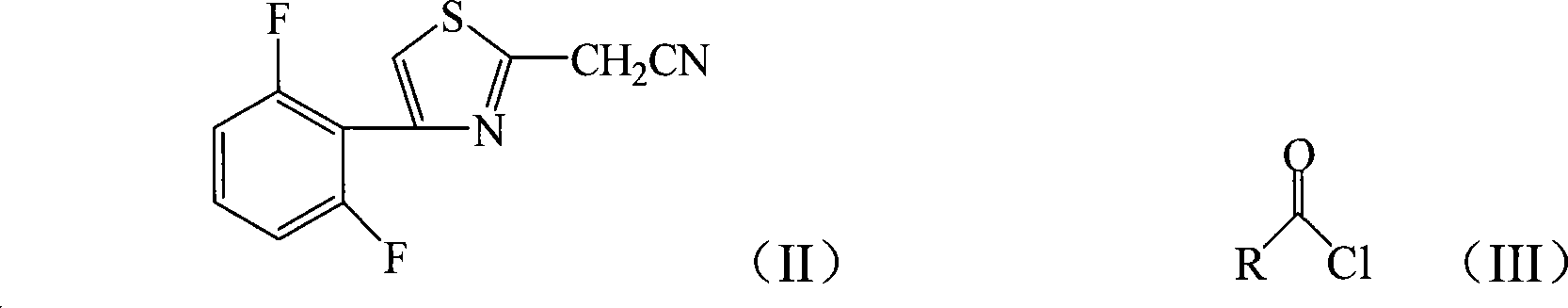2-thiazolylacrylonitrile compounds and its synthetic method and application
A technology of thiazolyl acrylonitrile and its synthesis method, which is applied in the fields of botany equipment and methods, applications, organic chemistry, etc., and can solve the problem of the limited application of 2-thiazolyl acrylonitrile compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 4.72g (20mmol) 2-cyanomethyl-4-(2,6-difluorophenyl) thiazole and 3.05g (30mmol) triethylamine to 20mL tetrahydrofuran, add dropwise 2.76g (24mmol) chloroacetyl chloride , 40min dripping, then reacted at 66°C for 12h, after the reaction was completed, filtered, and the filter cake was recrystallized with chloroform to obtain light green crystal 2-[4-(2,6-difluorophenyl)-2-thiazolyl]- 4.40g of 3-hydroxy-3-chloromethylacrylonitrile, melting point 193-195°C, yield 70%.
[0027] of the compound 1 H NMR and IR are as follows,
[0028] 1 HNMR (CDCl 3 )δ=4.43(H, s, CH 2 Cl), 7.09~7.13 (2H, m, Ar- CH -CH- CH ), 7.40 (H, s, Triazole), 7.42~7.50 (H, m, Ar-CH- CH -CH), 14.67 (H, OH) IR (KBr, cm -1 )3378, 3155, 2201, 1524, 1610~1450, 1233, 783, 727
Embodiment 2
[0030] 4.72g (20mmol) of 2-cyanomethyl-4-(2,6-difluorophenyl)thiazole and 1.13g (28 mmol) of sodium hydroxide were added to 25mL of 1,4-dioxane, and 2.10g of (26mmol) acetyl chloride, after 40 minutes of dripping, then reacted at 100°C for 6 hours, after the reaction was completed, filtered, and the filter cake was recrystallized with chloroform to obtain white flaky crystals 2-[4-(2,6-difluorophenyl)- 4.36 g of 2-thiazolyl]-3-hydroxy-3-methacrylonitrile, melting point 197-198°C, yield 78%.
[0031] of the compound 1 H NMR and IR are as follows,
[0032] 1 HNMR (CDCl 3 )δ=2.41(3H, s, CH 3 ), 7.05~7.09 (2H, m, Ar- CH -CH- CH ), 7.36~7.46 (2H, m, Ar-CH- CH -CH&Triazole) IR (KBr, cm -1 )3453, 3179, 2191, 1513, 1610~1450, 1378, 1246, 790, 712
Embodiment 3
[0034] Add 4.72g (20mmol) 2-cyanomethyl-4-(2,6-difluorophenyl) thiazole and 3.22g (30mmol) sodium carbonate to 20mL dichloromethane, add 2.27g (24 mmol) propane dropwise Acyl chloride, after 40 minutes of dripping, then reacted at 40°C for 12 hours, after the reaction was completed, filtered, and the filter cake was recrystallized with chloroform to obtain a light green solid 2-[4-(2,6-difluorophenyl)-2-thiazolyl] - 4.21 g of 3-hydroxy-3-ethylacrylonitrile, melting point 172-174°C, yield 72%. of the compound 1 H NMR and IR are as follows,
[0035] 1 HNMR (CDCl 3 ) δ = 1.25 ~ 1.28 (3H, t, CH 3 ), 2.70~2.75 (2H, m, CH 2 ), 7.05~7.09 (2H, t, Ar- CH -CH- CH ), 7.37~7.41 (2H, m, Ar-CH- CH -CH&Triazole), 14.67 (H, OH) IR (KBr, cm -1 )3450, 3161, 2194, 1513, 1620~1480, 1459, 1237, 997
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com