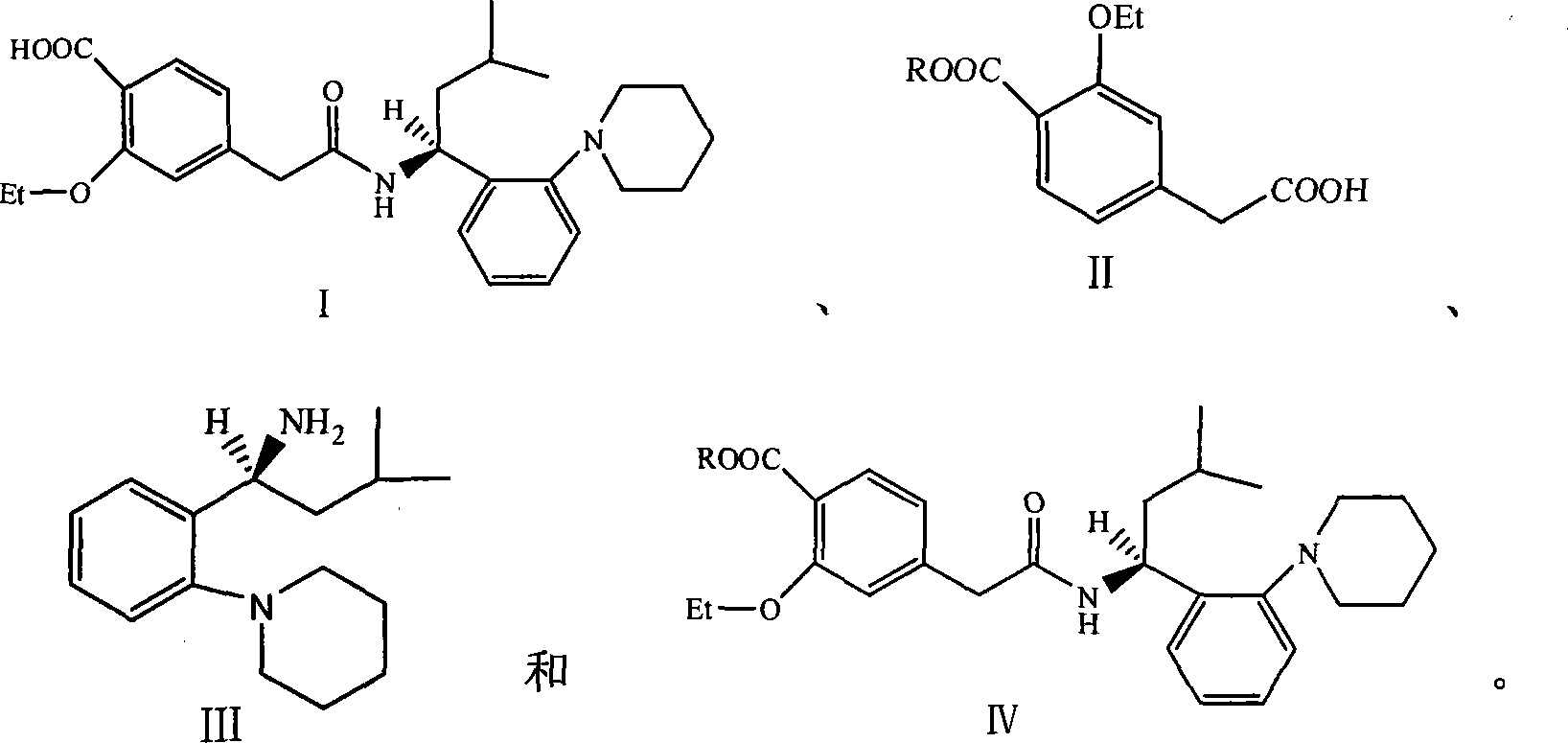
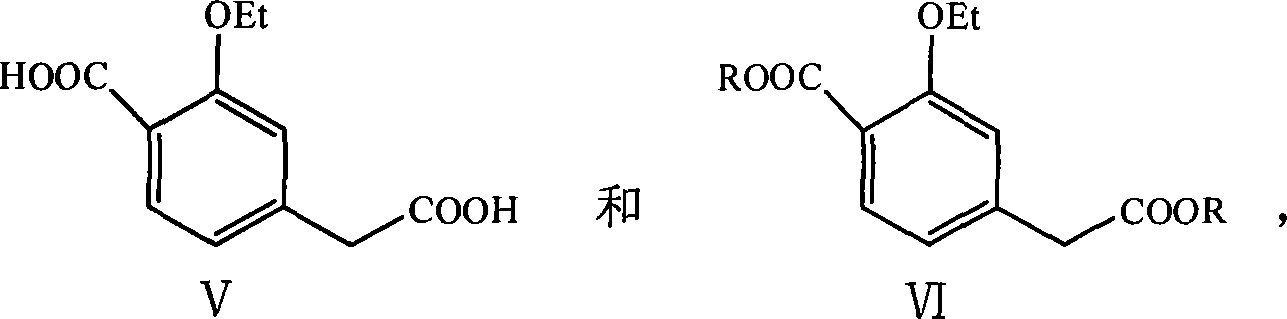
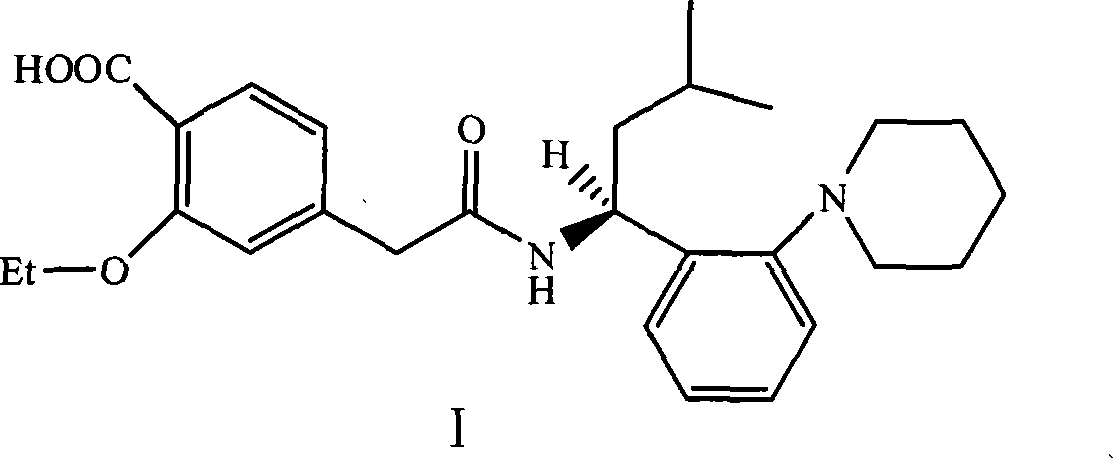
Method for producing repaglinide
A compound and catalyst technology, applied in the field of preparation of repaglinide and its intermediates, can solve the problems of high reaction temperature, low total yield, low reaction temperature, etc., and achieve high optical purity, environmental friendliness, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Synthesis of 2-ethoxyl group-4-(ethoxycarbonylmethyl) ethyl benzoate
[0041] Dissolve 4-carboxymethyl-2-ethoxybenzoic acid (90g, 0.401mol) in 900mL ethanol, add concentrated sulfuric acid (4g, 0.041mol), heat to reflux with stirring, and react for 8h. After completion of the reaction, concentrate, add 250 mL of dichloromethane to the residue to dissolve, wash twice with 500 mL of 5% sodium bicarbonate solution, and then wash with 200 mL×2 water. Dry over anhydrous sodium sulfate, filter with suction, and concentrate to obtain 99.6 g of ethyl 2-ethoxy-4-(ethoxycarbonylmethyl)benzoate, with a yield of 88.6%.
Embodiment 2
[0042] Example 2 Synthesis of 2-ethoxy-4-(ethoxycarbonylmethyl) ethyl benzoate
[0043] Dissolve 4-carboxymethyl-2-ethoxybenzoic acid (90g, 0.401mol) in 900mL ethanol, add SO 4 2- / TiO 2 Solid superacid (6g), heated to reflux with stirring, reacted for 8h. After the reaction was completed, filter with suction, concentrate, add 250 mL of dichloromethane to the residue to dissolve, wash twice with 500 mL of 5% sodium bicarbonate solution, and then wash with 200 mL×2 water. Dry over anhydrous sodium sulfate, filter with suction, and concentrate to obtain 98.7 g of ethyl 2-ethoxy-4-(ethoxycarbonylmethyl)benzoate, with a yield of 87.8%.
Embodiment 3
[0044] Example 3 Synthesis of 2-ethoxy-4-(methoxycarbonylmethyl)methyl benzoate
[0045] 4-Carboxymethyl-2-ethoxybenzoic acid (90g, 0.401mol) was dissolved in 900mL of methanol, concentrated sulfuric acid (4.5g, 0.0459mol) was added, heated to reflux under stirring, and reacted for 8h. After completion of the reaction, concentrate, add 250 mL of dichloromethane to the residue to dissolve, wash twice with 500 mL of 5% sodium bicarbonate solution, and then wash with 200 mL×2 water. Dry over anhydrous sodium sulfate, filter with suction, and concentrate to obtain 88.9 g of methyl 2-ethoxy-4-(methoxycarbonylmethyl)benzoate, with a yield of 87.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com