Preparation method of sildenafil
A technology for sildenafil and compounds, which is applied in the field of preparation of sildenafil, can solve the problems of difficult removal of by-products, no relevant reports, unstable intermediates, etc., and achieves easy control of reaction conditions, simple and convenient purification process, The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
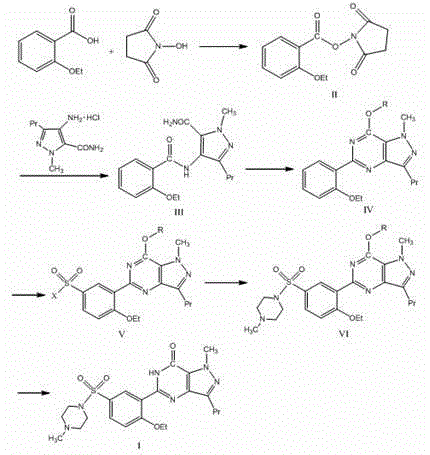
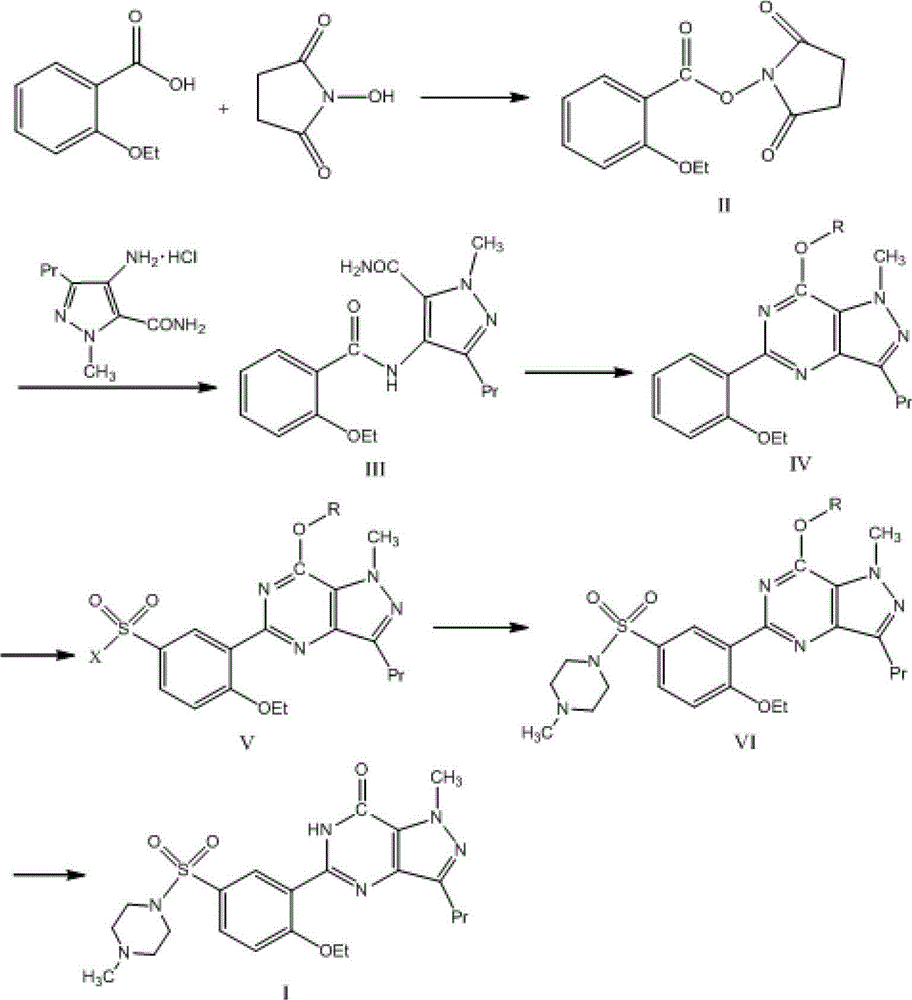
[0026] Example 1 Synthesis of N-(2-ethoxybenzoyl)succinimide (Compound II)
[0027] Add N,N'-Dicyclohexylcarbodiimide (DCC) (7.74g, 37.5mmol) and 30ml of dry dioxane solution to the dry reaction flask, stir to dissolve. Dissolve 2-ethoxybenzoic acid (4.99g, 30.0mmol) and N-hydroxysuccinimide (NHS) (3.80g, 33.0mmol) in 25ml of dry dioxane and add dropwise to In the above DCC solution. After the addition was completed, the reaction was stirred at room temperature for 1 h. After the reaction, 1.0 ml of water was added dropwise to the reaction solution, and stirring was continued for 1 hour. After filtering, the filtrate was concentrated to dryness to obtain a viscous liquid. The viscous liquid was dissolved in 30 ml of dichloromethane to obtain solution ①; the filter cake obtained by filtration was washed with dichloromethane (10 ml×3) to obtain washing liquid ②. Combine solution ① and lotion ②, wash with saturated brine (20ml×1), and wash with water (20ml×2). The organic phase ...
Embodiment 2
[0030] Example 2 Synthesis of 4-(2-ethoxybenzamide)-1-methyl-3-propyl-1H-pyrazole-5-carboxamide (Compound III)
[0031] Add 1-methyl-3-propyl-4-aminopyrazole-5-carboxamide hydrochloride (4.59g, 21.0mmol) hydrochloride to a dry three-neck flask, add 45ml of dry dichloromethane, and stir 30min. Add 4-dimethylaminopyridine (DMAP) (7.69 g, 63.0 mmol), stir at room temperature for 20 min, and then add compound II (6.90 g, 26.2 mmol). The reaction was stirred at room temperature for 1 hour, and heated to reflux for 24 hours. After the reaction, the reaction solution was naturally cooled to room temperature, diluted with 15ml of dichloromethane, adjusted to neutral with 0.5mol / L dilute hydrochloric acid, separated the organic layer, and washed with saturated brine (20ml×2), Wash with water (20ml×2). Dry with anhydrous magnesium sulfate, concentrate to near dryness, filter, and wash the filter cake with dichloromethane (6ml×2). The filter cake was vacuum dried to obtain 4.85 g of yel...
Embodiment 3
[0035] Example 3 1-Methyl-3-n-propyl-5-(2-ethoxyphenyl)-7-methoxy-1,6-dihydro-7H-pyrazolo[4,3-d] Synthesis of Pyrimidine (Compound IV)
[0036] Weigh phosphorus pentachloride (6.04g, 29.0mmol) and add it to 24.0ml of phosphorus oxychloride. Compound III (4.78 g, 14.5 mmol) was added to a dry three-necked flask. Under the protection of nitrogen, the mixed solution of phosphorus pentachloride and phosphorus oxychloride was added dropwise with stirring, and the temperature was about 2°C. After dripping, the temperature was raised to 90°C and reacted for 4h. After finishing the reaction, the reaction solution was cooled to room temperature and then added dropwise to crushed ice under stirring. A yellow solid was formed. Add dichloromethane to extract (40ml×3) the above solids, combine the extracted dichloromethane solutions, wash with saturated brine (40ml×4), and dry with anhydrous magnesium sulfate for 2h. Filter, collect the filtrate, and concentrate under reduced pressure at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com