Novel conductive agent doping/coating lithium iron phosphate material and its production method
A technology of lithium iron phosphate and conductive agent, which is applied in chemical instruments and methods, electrode manufacturing, circuits, etc., can solve the problems of lithium iron phosphate performance impact, electrochemical capacity loss, and poor high-rate discharge performance, so as to improve discharge and discharge performance. The effects of cycle stability, high-rate charge-discharge performance, high conductivity and bulk density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Weigh Fe(NO 3 ) 3 9H 2 O 4.04g, NH 4 h 2 PO 4 1.15g, respectively dissolved in 100mL deionized water, added dropwise and mixed, under stirring, 1 mol / L ammonia water was added at 60°C until the pH of the solution was 5, filtered, washed and dried at 80°C. The resulting precursor and 0.388g Li 2 CO 3 , 0.079gCoS 2 Mix and grind, put into porcelain crucible. at a velocity of 1dm 3 min -1 99.999% argon flow, at 3°C·min -1Raise the temperature to 350°C at the same rate, keep it for 4 hours, cool down to room temperature naturally, grind and press into tablets, then raise the temperature to 500°C at the same rate, and keep it for 18 hours. Naturally cool to room temperature with the furnace, take out the product and grind to obtain the doped lithium iron phosphate.
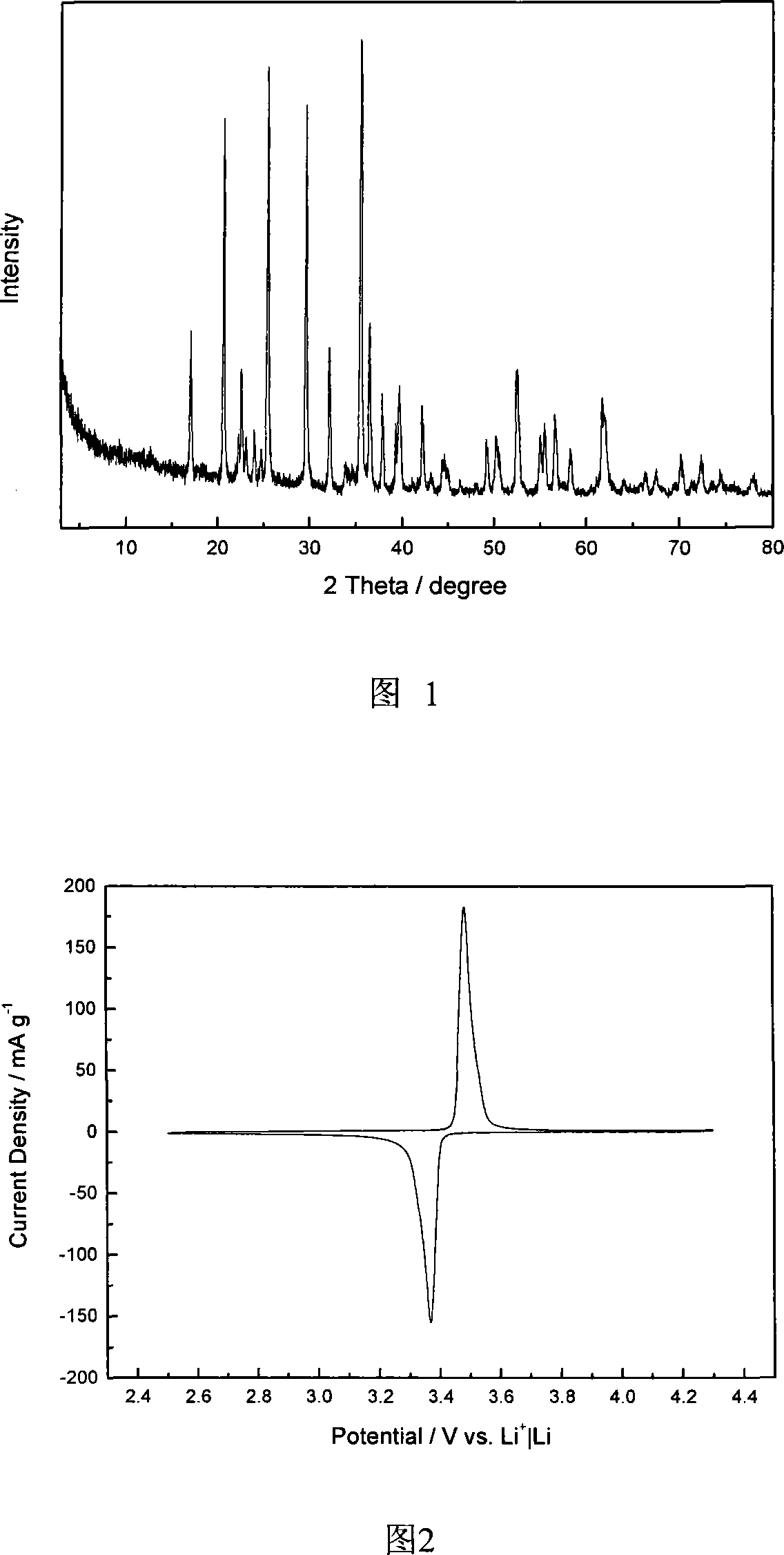
[0042] The XRD of the product is shown in Figure 1. It can be seen from Figure 1 that olivine-type lithium iron phosphate, CoS 2 The coating did not change its crystal form, and there were no other...
Embodiment 2
[0044] Weigh Fe(NO 3 ) 3 9H 2 O 4.04g, NH 4 h 2 PO 4 1.15 g, respectively dissolved in 100 mL deionized water, added dropwise and mixed, added 1 mol / L ammonia water at 60°C under stirring until the pH value of the solution was 5, filtered, washed and dried at 80°C. The resulting precursor and 0.388g Li 2 CO 3 , mixed and ground, put into a porcelain crucible, at a flow rate of 1dm 3 min -1 99.999% argon flow, at 3°C·min -1 Raise the temperature to 350°C at the same rate, keep it for 4 hours, cool down to room temperature naturally, grind and press into tablets, then raise the temperature to 700°C at the same rate, and keep it for 18 hours. Naturally cool to room temperature with the furnace, take out the product and grind finely to obtain lithium iron phosphate. Weigh 0.0607g Co(AC) 2 4H 2 O and 0.0927g NH 2 CSNH 2 Dissolve in 20mL of ethanol, add 0.3g of the prepared lithium iron phosphate, disperse by ultrasonic wave for 0.5 hours, then place the mixture in a ...
Embodiment 3
[0046] Weigh Fe(NO 3 ) 3 9H 2 O 4.04g, NH 4 h 2 PO 4 1.15 g, respectively dissolved in 100 mL deionized water, added dropwise and mixed, added 1 mol / L ammonia water at 60°C under stirring until the pH value of the solution was 5, filtered, washed and dried at 80°C. The resulting precursor and 0.388g Li 2 CO 3 , mixed and ground, put into a porcelain crucible, at a flow rate of 1dm 3 min -1 99.999% argon flow, at 3°C·min -1 Raise the temperature to 350°C at the same rate, keep it for 4 hours, cool down to room temperature naturally, grind and press into tablets, then raise the temperature to 700°C at the same rate, and keep it for 18 hours. Naturally cool to room temperature with the furnace, take out the product and grind finely to obtain lithium iron phosphate. Weigh 0.1128g SnCl 2 and 0.0435g CTAB were dissolved in 25mL of water, 0.3g of the prepared lithium iron phosphate was added, ultrasonically dispersed for 0.5 hours, and 0.398mL of 6.5mol L was added dropwi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com