Co-polymer based polymer electrolyte material for lithium battery, compound electrolyte film and its preparation method
A technology of electrolyte materials and polymers, which is applied in the manufacture of electrolyte batteries, batteries with organic electrolytes, non-aqueous electrolyte batteries, etc., can solve problems such as safety, hidden dangers, and limited room temperature conductivity improvement, and achieve the effect of improving conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
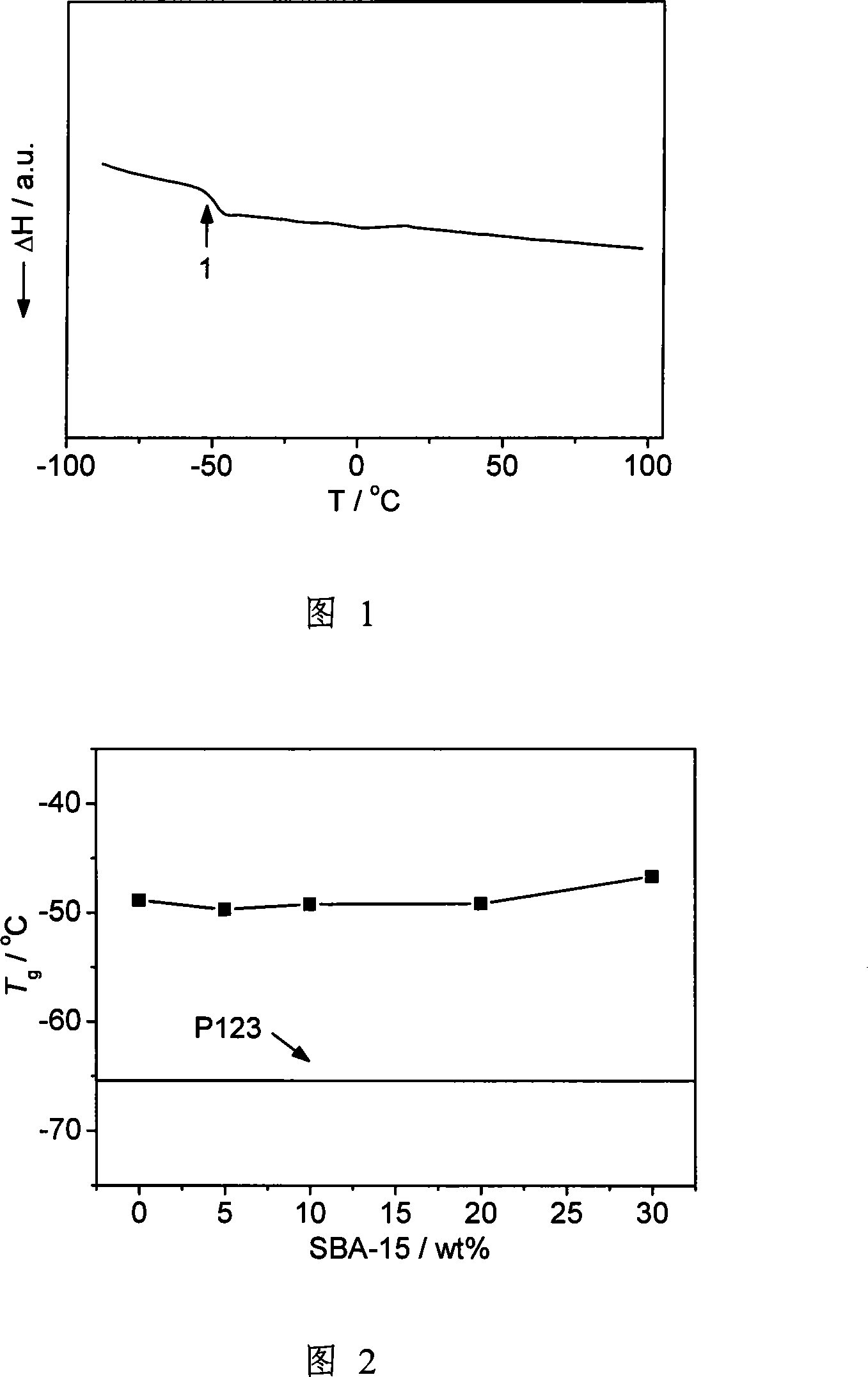
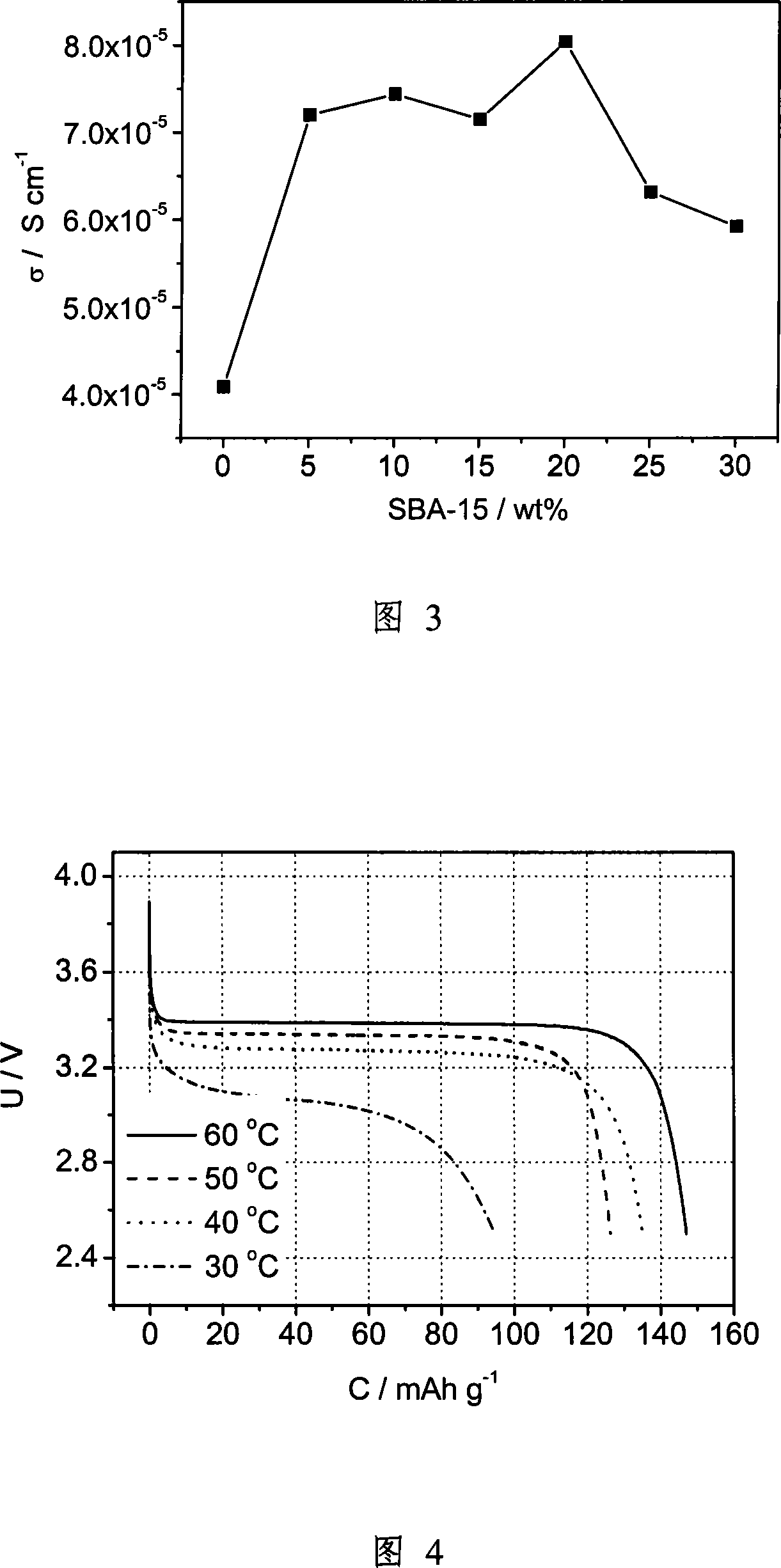
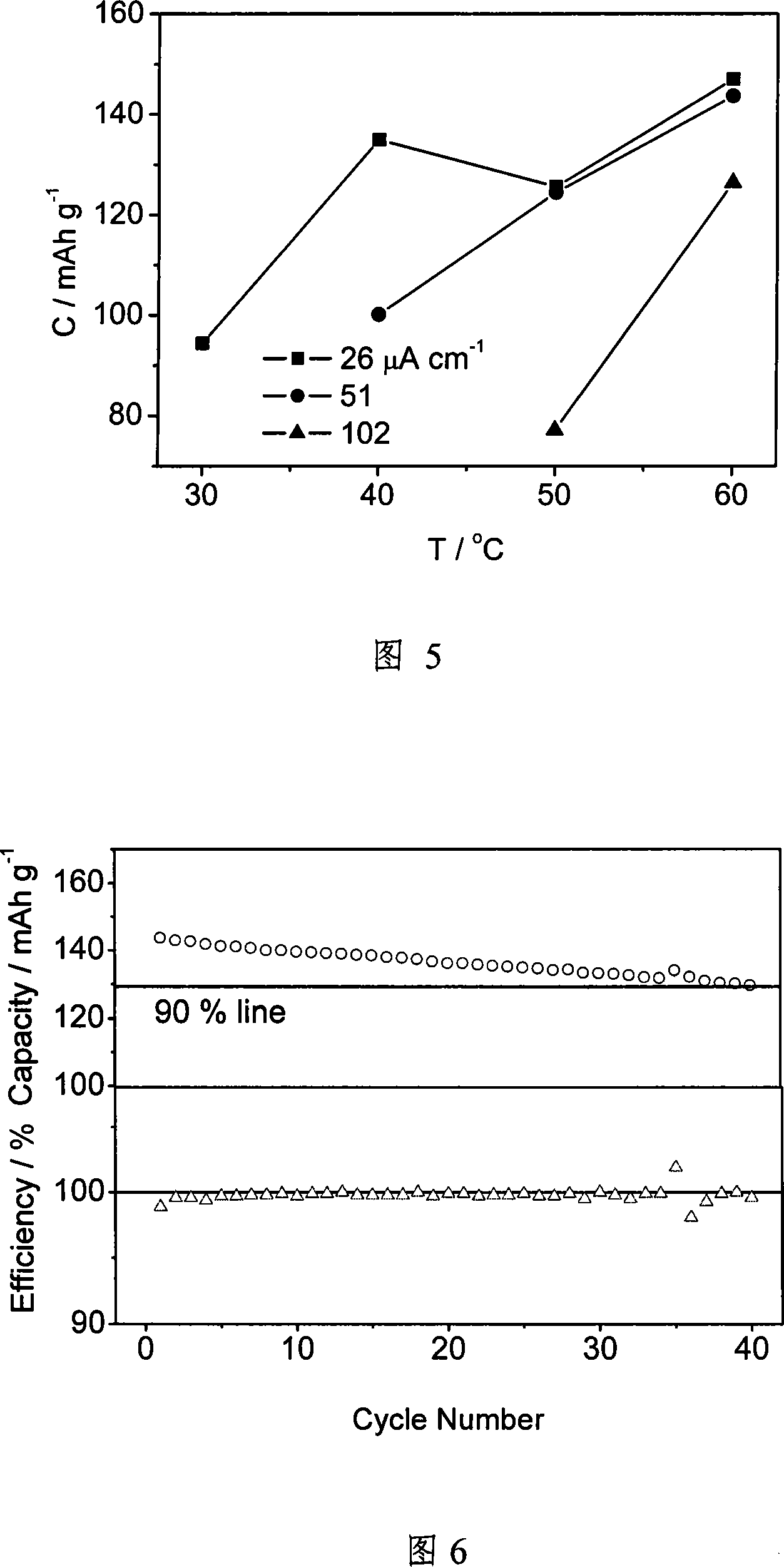
[0041] (a) Using mesoporous SiO 2 , first calcined at 500 ° C for 6 hours to remove the template in the channel, referred to as SBA-15, and its particle size is about 1 micron. Prepare 1M LiTFSI / (ethylene carbonate+propylene carbonate) liquid electrolyte, wherein the weight percentage of ethylene carbonate and propylene carbonate is 1:1. Soak SBA-15 powder in liquid electrolyte in a glove box. Soaking time is 4 days. The solution was then filtered.
[0042] (b) Weigh 0.5 g of EO 20 -PO 70 -EO 20Copolymer, referred to as P123. Weigh 0.170 g of LiTFSI (the molecular weight of LiTFSI is 287.08, the molecular weight of P123 is 5800, contains 110 O atoms, the average molecular weight is 580 / 11, and the molar ratio is 16). According to 0%, 5%, 10%, 15%, 20%, 25%, 30% of the total weight percentage of the copolymer and lithium salt, the SBA-15 powder soaked in liquid electrolyte is weighed, and these three are added to 5ml In acetonitrile, stir for 12 hours, and use ultrasoni...
Embodiment 2
[0046] Weigh 0.5 g EO 20 -PO 70 -EO 20 copolymer. LiTFSI was weighed according to O / Li molar ratio of 7, 8, 10, 12, 16, 20, 25, 40. These two were added to 5 ml of acetonitrile and stirred for 12 hours. Then, the solvent was evaporated in a glove box for 12 hours, and further dried in a vacuum oven at 80° C. for 5 hours.
[0047] Figure 7 shows the variation of ionic conductivity with LiTFSI concentration at 30 °C. Figure 8 shows the differential thermal curves of the polymer electrolyte material with an O / Li molar ratio of 16. Figure 9 shows the glass transition temperature T of the samples g Variation with LiTFSI concentration. It can be seen from the figure that the conductivity reaches a maximum value around n=20, which is 4.6×10 -5 Scm -1 . Moreover, this electrolyte material does not crystallize and has a low T g , indicating that its chain segment activity is strong.
Embodiment 3
[0049] Weigh 0.5 g EO 20 -PO 70 -EO 20 copolymer. Weigh LiClO according to the O / Li molar ratio of 6, 7, 8, 10, 12 4 . These two were added to 5 ml of acetonitrile and stirred for 12 hours. Then, the solvent was evaporated in a glove box for 12 hours, and further dried in a vacuum oven at 80° C. for 5 hours.
[0050] Figure 10 shows the ionic conductivity at 30 °C versus LiClO 2 changes in concentration. Figure 11 shows the differential thermal curves of polymer electrolyte materials with an O / Li molar ratio of 8. Figure 1 2 shows the glass transition temperature T of the polymer electrolyte material g With LiClO 4 change in concentration. It can be seen from the figure that the conductivity reaches a maximum value around n=8, which is 8.9×10 -6 Scm -1 . Moreover, this electrolyte material does not crystallize and has a low T g , indicating that its chain segment activity is strong.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com