Method for preparing nano Fe3O4
A nano and powder technology, applied in the nano field, achieves the effects of low cost, good dispersion and strong practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
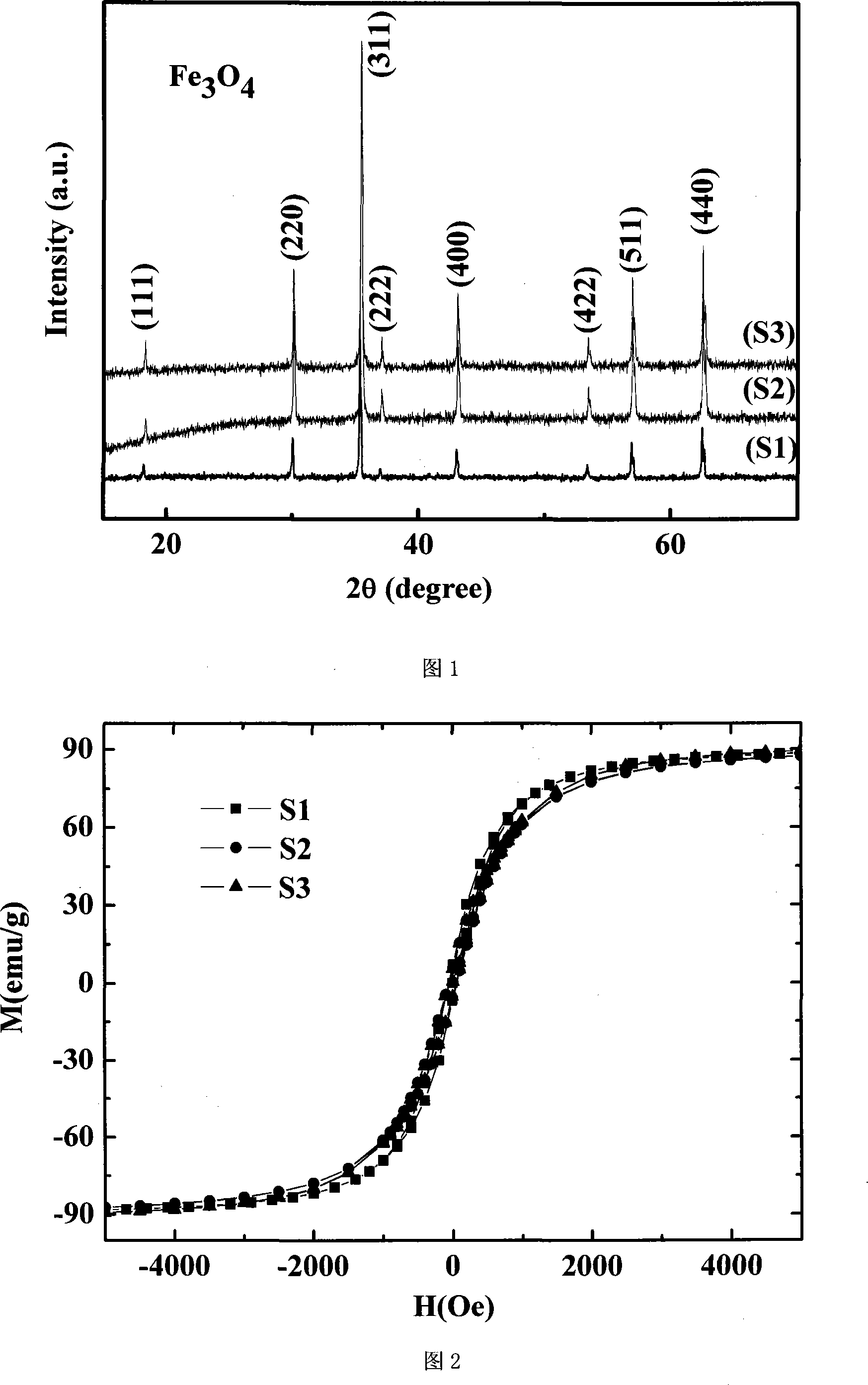
[0023] Utilize the present invention, under air environment, 40 ℃, the Fe(NO 3 ) 3 9H 2 O and C 6 h 8 o 7 ·H 2 O was dissolved in 50ml deionized water, where Fe 3+ The molar concentration is 0.4mol / l. Put the prepared mixed solution into a water bath for ultrasonic stirring for 30 minutes, and then evaporate in a 75°C water bath until it turns into a transparent colloid. The obtained wet gel was further dried in an oven set at 130°C for 45 minutes to remove the remaining moisture, and then the gel from which the remaining moisture was removed was quickly placed on a resistance furnace to heat until the gel began to self-propagate and burn. Then grind the fluffy material obtained by self-propagating combustion for 30 minutes to obtain a powder, press the powder into a block and place it in an atmosphere furnace, and finally sinter at 650°C for 10 hours in an argon atmosphere to obtain nano-Fe 3 o 4 Block sample S1. The XRD diffraction pattern, hysteresis loop diagram ...
Embodiment 2
[0025] Fe(NO 3 ) 3 9H 2 O and C 6 h 8 o 7 ·H 2 O was dissolved in 40ml deionized water, where Fe 3+ The molar concentration is 0.5mol / l. The prepared mixed solution was placed in a water bath for ultrasonic stirring for 30 minutes, and then evaporated in a water bath at 77°C until it turned into a transparent colloid. The obtained wet gel was further dried in an oven set at 130°C for 35 minutes to remove the remaining moisture, and then the gel from which the remaining moisture was removed was quickly placed on a resistance furnace to heat until the gel began to self-propagate and burn. Then grind the fluffy material obtained by self-propagating combustion for 30 minutes to obtain a powder, put the powder in an atmosphere furnace, and finally sinter at 670°C for 11 hours in an argon atmosphere to obtain nano-Fe 3 o 4 Powder sample S2. The XRD diffraction pattern, hysteresis loop diagram and scanning electron micrograph of sample S2 are shown in Figure 1, Figure 2 and...
Embodiment 3
[0027] Fe(NO 3 ) 3 9H 2 O and C 6 h 8 o 7 ·H 2 O was dissolved in 20ml deionized water, where Fe 3+ The molar concentration is 1mol / l. The prepared mixed solution was placed in a water bath for ultrasonic stirring for 40 minutes, and then evaporated in a water bath at 85°C until it turned into a transparent colloid. The obtained wet gel was further dried in an oven set at 130°C for 30 minutes to remove the remaining moisture, and then the gel from which the remaining moisture was removed was quickly placed on a resistance furnace to heat until the gel began to self-propagate and burn. Then grind the fluffy material obtained by self-propagating combustion for 30 minutes to obtain a powder, put the powder in an atmosphere furnace, and finally sinter at 680°C for 12 hours in an argon atmosphere to obtain nano-Fe 3 o 4 Powdered sample S3. The XRD diffraction diagram, hysteresis loop diagram and scanning electron micrograph diagram of sample S3 are shown in Fig. 1 , Fig. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com