Kukoline or its salts sustained-release microsphere and preparation thereof and sustained-release injection preparations
A technology for sustained-release microspheres and injection preparations, which is applied in the directions of medical preparations, anti-inflammatory agents, and pharmaceutical formulations containing active ingredients to achieve long-lasting anti-inflammatory and analgesic effects, short process flow, and good reproducibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
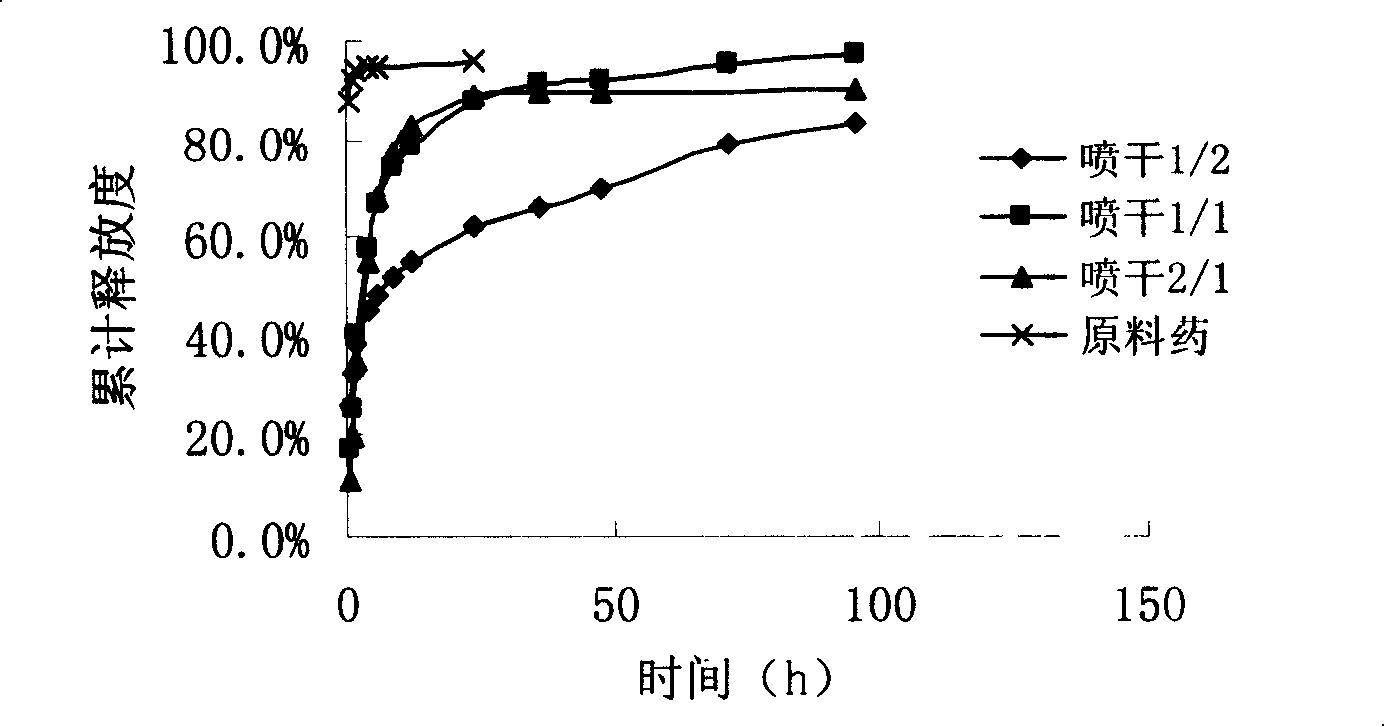
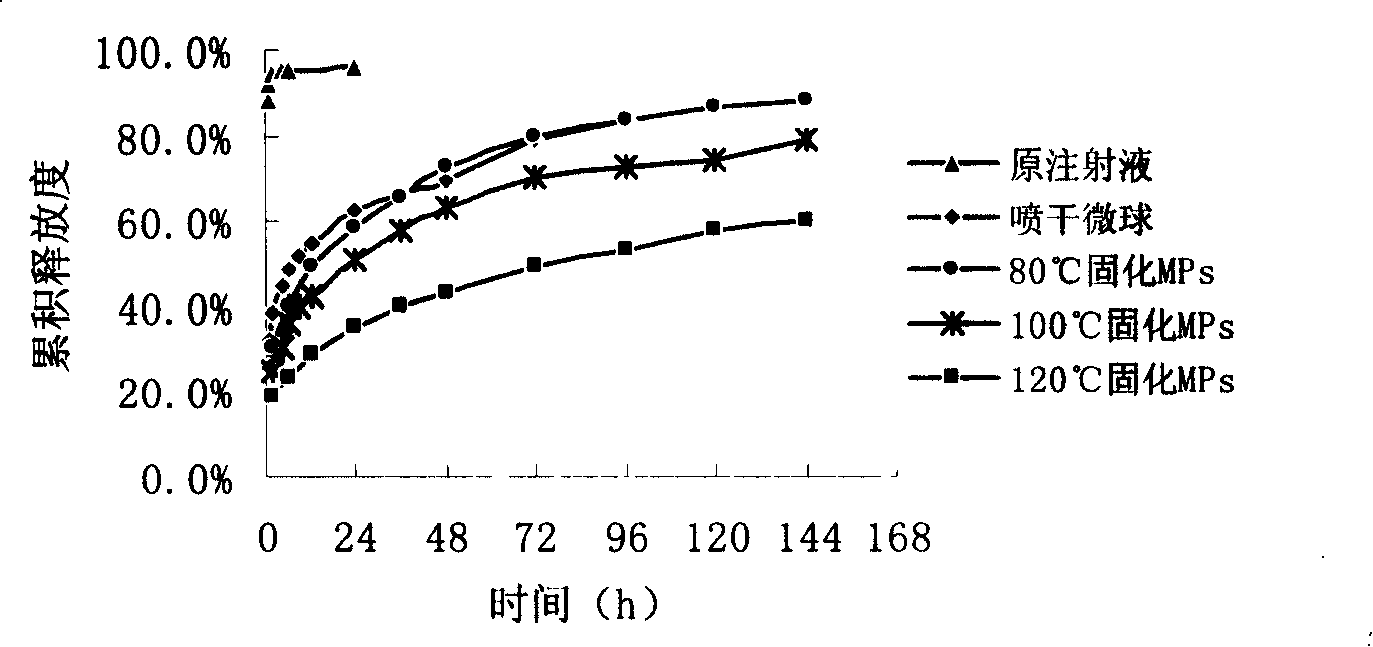
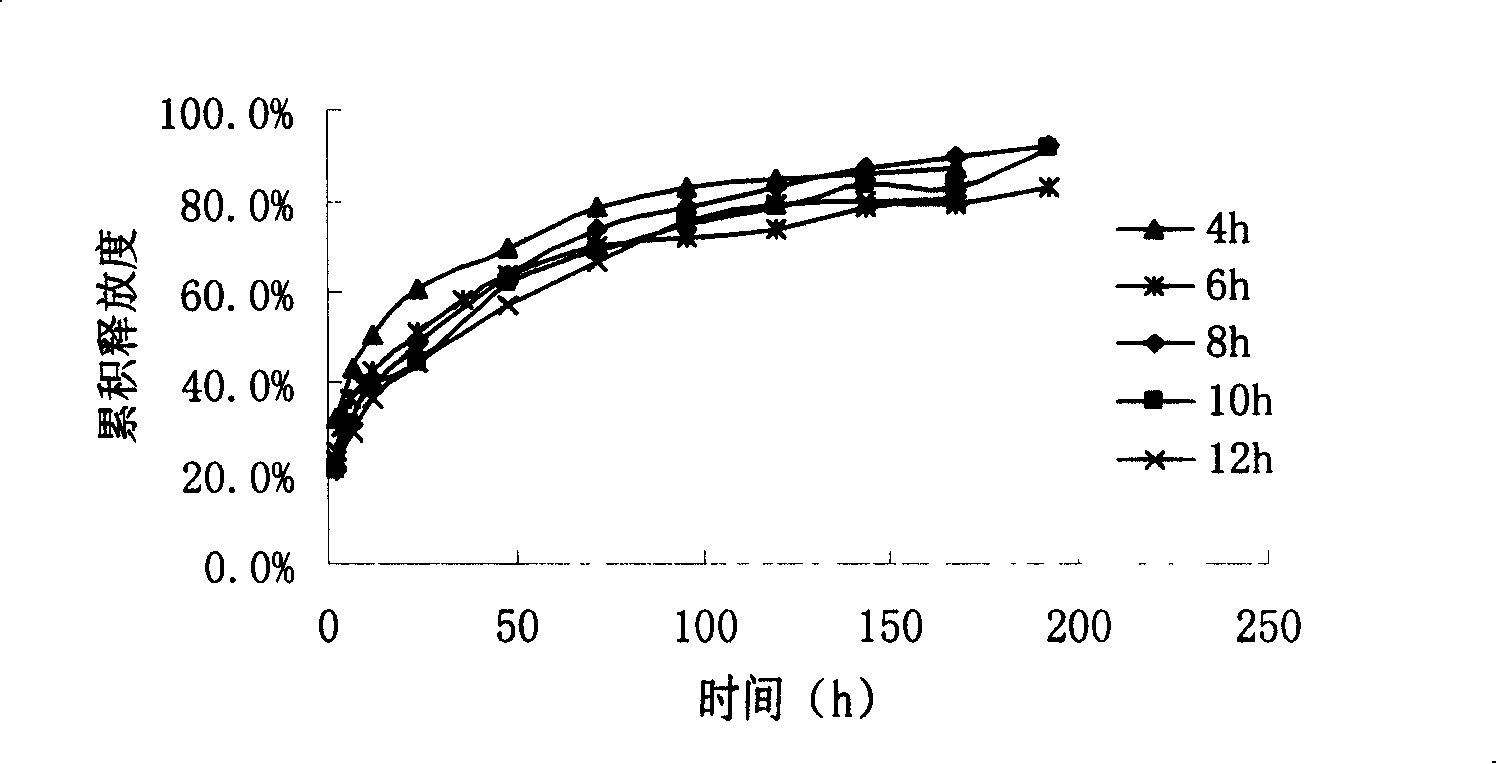
Image
Examples
Embodiment 1
[0021] Take 1.0 g of sinomenine hydrochloride, 2.0 g of bovine serum albumin, add 100 ml of water to fully dissolve it, and filter to obtain a clear solution. The solution is spray-dried with a spray drier, and the obtained white powder is placed in a silica gel drier at room temperature for 3 days to obtain drug-loaded albumin sustained-release microspheres. Take 100 mg of microspheres, add 2 ml of soybean oil for injection, and ultrasonicate for 30 seconds to fully suspend the microspheres to obtain the sustained-release injection preparation of sinomenine hydrochloride of the present invention.
Embodiment 2
[0023] Take 2.0 g of sinomenine hydrochloride and 2.0 g of bovine serum albumin, add 100 ml of water to fully dissolve them, and then filter to obtain a clear solution. The solution was spray-dried with a spray dryer, and the obtained white powder was heated and solidified in an oven at 120° C. for 4 hours, and then cooled to room temperature to obtain drug-loaded albumin sustained-release microspheres. Take 75 mg of microspheres, add 2 ml of sesame oil for injection, and ultrasonicate for 30 seconds to fully suspend the microspheres to obtain the slow-release injection preparation of sinomenine hydrochloride of the present invention.
Embodiment 3
[0025] Take 4.0 g of sinomenine hydrochloride and 2.0 g of human serum albumin, add 100 ml of water to make them fully dissolved, and then filter to obtain a clear solution. The solution is spray-dried with a spray dryer, and the obtained white powder is heated and solidified in an oven at 100° C. for 8 hours, and then cooled to room temperature to obtain drug-loaded albumin sustained-release microspheres. Take 50 mg of microspheres, add 0.2 ml of polyethylene glycol 200, 1.8 ml of water for injection, and ultrasonicate for 30 seconds to fully suspend the microspheres to obtain the slow-release injection preparation of sinomenine hydrochloride of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com