Orange reactive dyestuffs and preparation method thereof
A reactive dye, orange technology, applied in the field of orange reactive dye and its preparation, can solve the problems of difficult environmental treatment, dark color of dyeing residue, poor fabric feel, etc., and achieves excellent solubility, reduced difficulty, and good social benefits. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
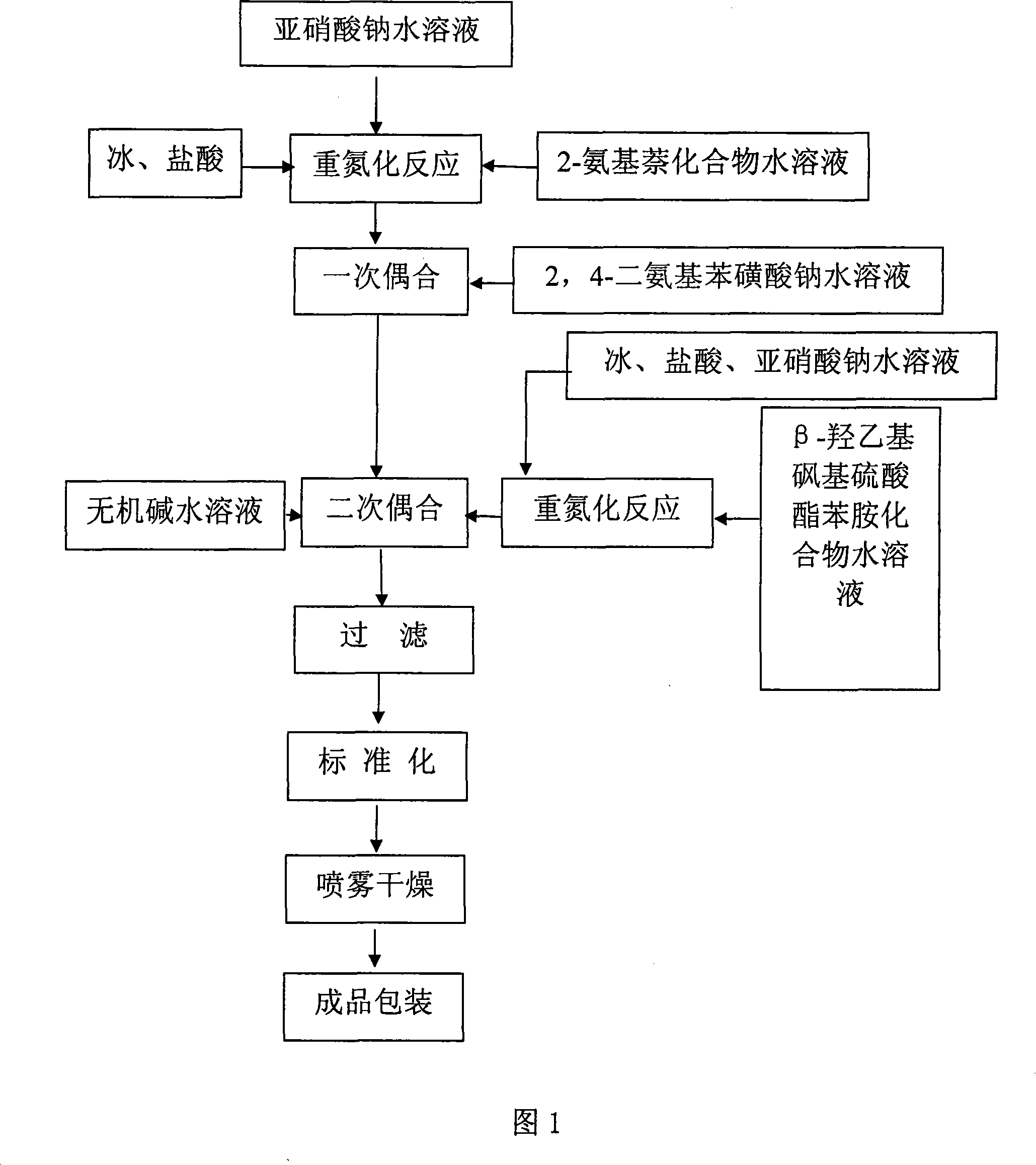
[0040] As shown in Figure 1, the preparation method of orange reactive dye of the present invention comprises the following steps:
[0041] a. Diazotization reaction: add 2-naphthylamine-disulfonic acid in water, stir and make a slurry at room temperature, add ice, add hydrochloric acid and sodium nitrite aqueous solution at a temperature of 0-10°C, and keep the pH of the reaction liquid = 1-3, slight excess of nitrous acid, stirring and reacting for 2 hours, after the reaction, remove the remaining nitrous acid;
[0042] b. Primary coupling: add sodium 2,4-diaminobenzenesulfonate to water, stir to dissolve at room temperature, add the diazotization reaction feed solution of 2-naphthylamine-disulfonic acid obtained in step a, and keep the pH under stirring =1-4, temperature 5-17°C, coupling reaction for 1 hour, and the end point of the first coupling is detected by a spectrophotometer;
[0043] c. Diazotization reaction: add β-hydroxyethyl sulfone sulfate aniline in water, st...
Embodiment 1
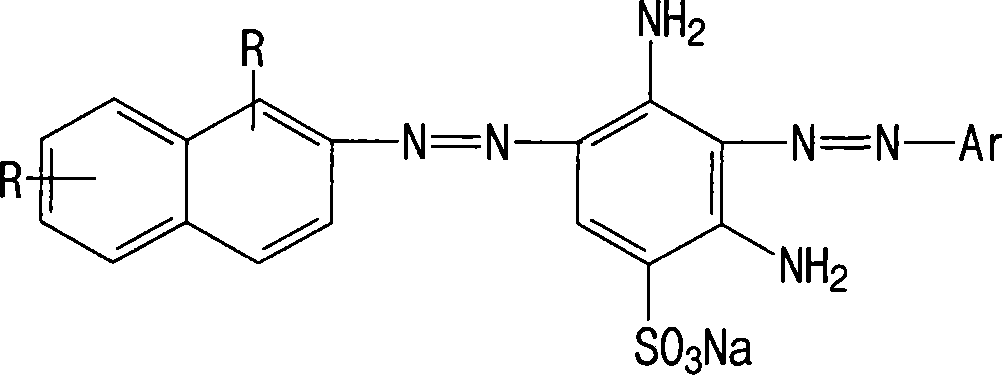
[0050] Reactive orange dye structural formula:
[0051]
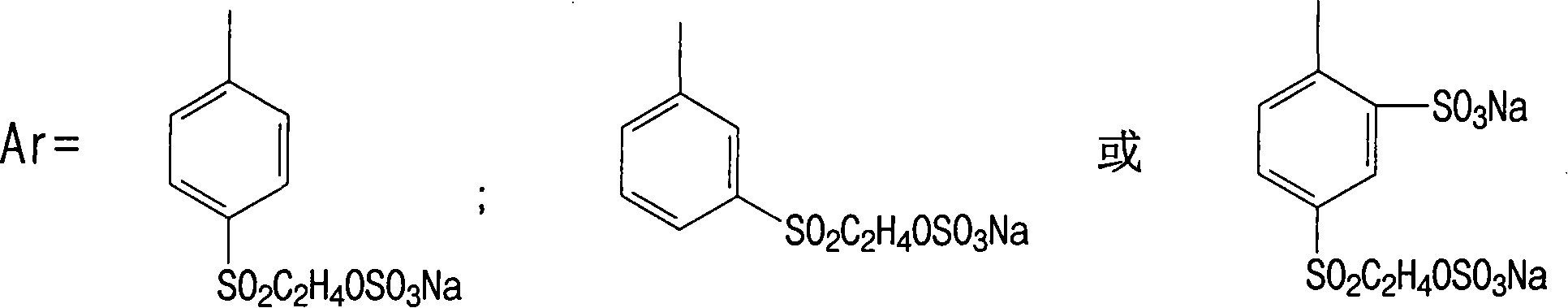
[0052] in:
[0053] or
[0054] The preparation method is as follows:
[0055] Take 2-naphthylamine-1,5-disulfonic acid and p-beta-hydroxyethylsulfone sulfate aniline as diazo components for illustration, if m-beta-hydroxyethylsulfone sulfate aniline is another If one diazo component is used, the material dissolution method and the amount of hydrochloric acid may be different, and the rest of the preparation methods are similar.
[0056] 1. Raw material ratio:
[0057] raw material name
The molar ratio of
100% usage
water(t)
Sodium 2,4-diaminobenzenesulfonate
210.2
1.00
210.2
0.7
2-Naphthylamine-1,5-disulfonic acid
303.3
1.01
306.3
0.7
β-Hydroxyethylsulfone sulfate aniline
281.3
1.01
284.1
0.7
69
2.03
140.2
0.468
hydrochloric aci...
Embodiment 2
[0073] Reactive orange dye has the following general structural formula:
[0074]
[0075] The preparation method is as follows:
[0076] Take 2-naphthylamine-4,8-disulfonic acid and 2-amino-5-β-hydroxyethylsulfone sulfate sodium benzenesulfonate as diazo components for illustration.
[0077] (1) Raw material ratio:
[0078] raw material name
The molar ratio of
100% usage
water(t)
Sodium 2,4-diaminobenzenesulfonate
210.2
1.00
210.2
0.7
2-Naphthylamine-4,8-disulfonic acid
303.3
1.01
306.3
0.8
2-Amino-5-β-hydroxyethylsulfone sulfuric acid
361.4
1.01
365
0.7
69
2.03
140.2
0.468
36.5
3.53
129
--
106
Based on pH
84
Based on pH
ice ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com