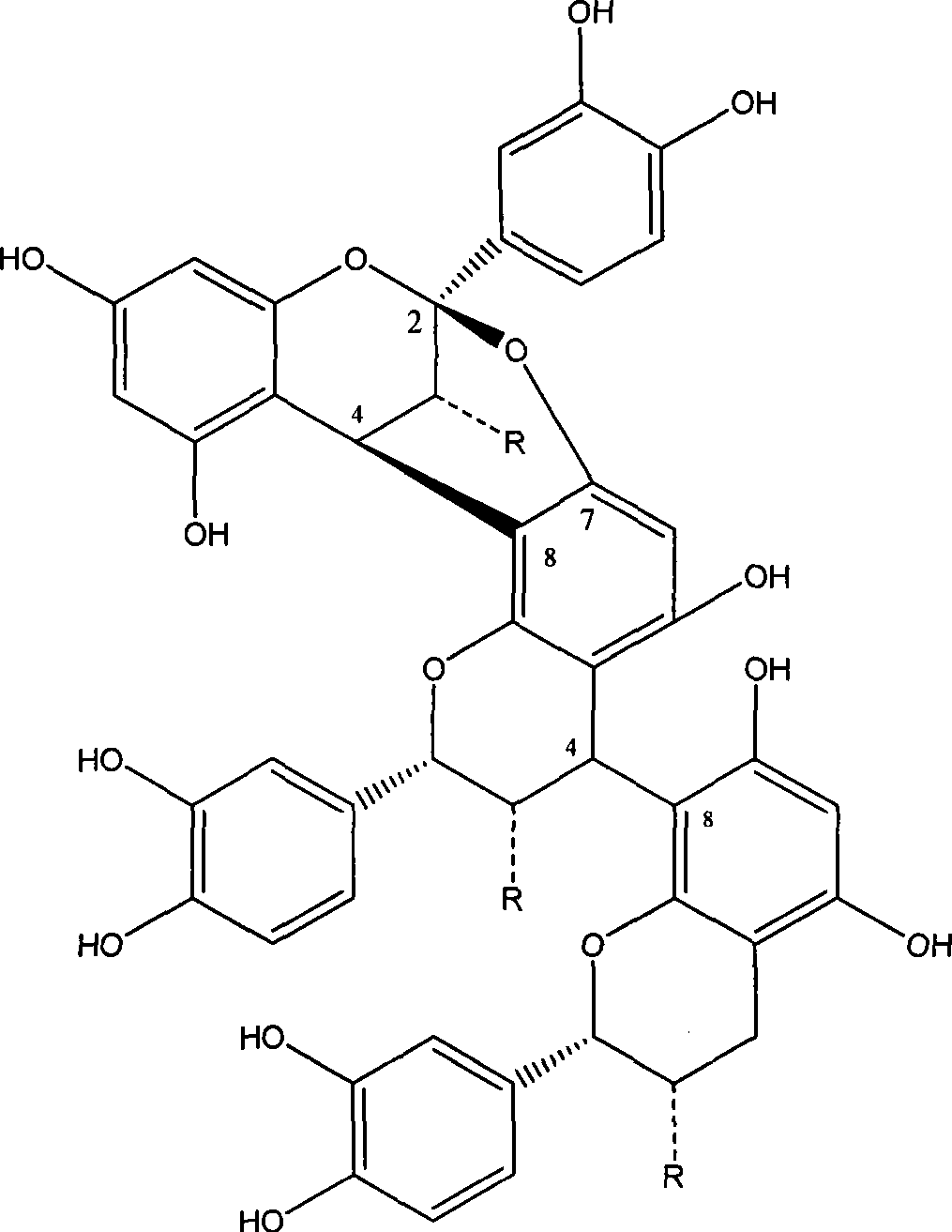
Method for catalytic hydrogenolysis of cinnamon proanthocyanidins high polymer to oligomer
A technology of proanthocyanidins and catalytic hydrogenolysis, applied in the direction of organic chemistry, etc., can solve the problems such as the degradation method of cinnamon high-polymer procyanidins, etc., and achieve the effect of improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Get 2.63g of cinnamon proanthocyanidin high polymer and dissolve it in 150ml volume percent concentration of 70% ethanol solution, put it into a reaction kettle, add 0.45g 10% palladium / carbon catalyst, and feed high-purity H 2 , under the condition of temperature of 100°C, pressure of 3.5MPa and stirring speed of 400rpm, the catalytic reaction was carried out for 200min. After the reaction is over, reduce the temperature of the reactor to room temperature, and slowly empty the H 2 , take out the reaction solution, filter, and recover the catalyst. The filtrate was concentrated under reduced pressure, ethanol was evaporated, and vacuum-dried to obtain 2.36 g of crude product with a yield of 89.7% and an average degree of polymerization of 2.82.
[0021] Dissolve the above crude product in water, put it on the LSA-21 macroporous resin column, wash it with 3 column volumes of distilled water, then elute with 3 column volumes of 50% ethanol solution, and concen...
Embodiment 2
[0022] Embodiment 2: Get 3.15 g of cinnamon proanthocyanidin high polymer and dissolve it in 150 ml volume percent concentration of 70% ethanol solution, put it into a reaction kettle, add 0.30 g of 10% palladium / carbon catalyst, and pass into high-purity H 2 , under the condition of temperature of 90°C, pressure of 3.0MPa and stirring speed of 400rpm, the catalytic reaction was carried out for 200min. After the reaction is over, reduce the temperature of the reactor to room temperature, and slowly empty the H 2 , take out the reaction solution, filter, and recover the catalyst. The filtrate was concentrated under reduced pressure, ethanol was evaporated, and vacuum-dried to obtain 2.75 g of product with a yield of 87.3% and an average degree of polymerization of 3.32.
[0023] Take the above dried crude product and dissolve it in water, put it on the LSA-21 macroporous resin column, wash it with 2 column volumes of distilled water, then elute with 3 column volumes of 60% eth...
Embodiment 3
[0024] Embodiment three: get cinnamon proanthocyanidin high polymer 4.50g and be dissolved in 150ml volume percentage concentration and be in the ethanol solution of 70%, pack in the reactor, add 0.60g 10% palladium / carbon catalyst, pass into high-purity H 2 , under the condition of temperature of 100°C, pressure of 3.5MPa and stirring speed of 400rpm, the catalytic reaction was carried out for 240min. After the reaction is over, reduce the temperature of the reactor to room temperature, and slowly empty the H 2 , take out the reaction solution, filter, and recover the catalyst. The filtrate was concentrated under reduced pressure, ethanol was evaporated, and vacuum-dried to obtain 4.17 g of crude product with a yield of 92.7% and an average degree of polymerization of 2.72.
[0025] Take the above dry crude product and dissolve it in water, put it on the LSA-21 macroporous resin column, wash it with 3 column volumes of distilled water, then elute with 4 column volumes of 70%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com