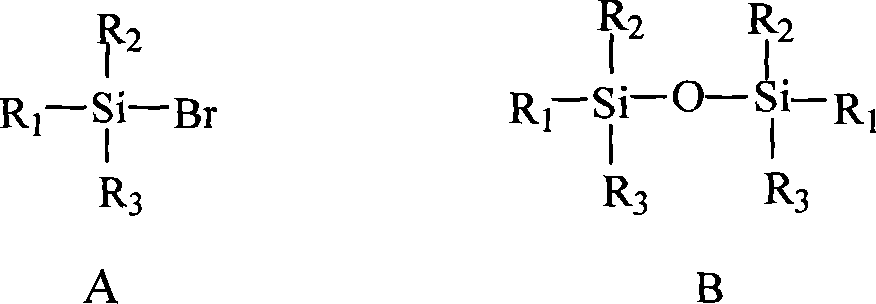Modified method for preparing trialkyl bromosilane
A technology of phosphorus tribromide and aluminum trichloride, applied in chemical instruments and methods, compounds of group 4/14 elements of the periodic table, organic chemistry, etc., can solve the problems of low atom utilization, environmental pollution, and preparation costs advanced questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The main steps of said preparation method are: at 20 DEG C~40 DEG C and under the existence condition that catalyst is arranged, phosphorus tribromide, hexamethyldisiloxane and 1,3-dimethylimidazolinone (solvent ) in a reactor, react at 60°C to 170°C (preferably 90°C to 110°C) for 4-12 hours (preferably 6-8 hours), stop the reaction, filter after cooling (filter out by-product red phosphorus), and the filtrate Atmospheric distillation is carried out, and the fraction at 80°C to 82°C is collected, which is bromotrimethylsilane.
[0032] The resulting distillation raffinate (mainly reaction solvent and catalyst) can be recycled, that is, add hexamethyldisiloxane and phosphorus tribromide to the distillation raffinate, and repeat the above-mentioned operation steps to obtain the target object (three bromomethylsilane).
[0033] Wherein: said catalyst is zinc chloride or / and aluminum trichloride, and the consumption of catalyst is 0.5wt%~5wt% (preferably 1wt%~2wt%) of hexa...
Embodiment 1
[0040] (1) Add hexamethyldisiloxane (industrial product, produced by Jiangsu Meilan Chemical Co., Ltd. , 55g, 0.34mol), phosphorus tribromide (industrial product, produced by Yixing Hengchang Chemical Co., Ltd., 187g, 0.67mol) and zinc chloride (industrial product, Shanghai Jiuying Chemical Co., Ltd., 0.7g), after adding, Under stirring, heat up to 95-105°C for 8 hours. Gas chromatography analysis shows that the hexamethyldisiloxane has completely reacted. After the reaction is completed, cool to ambient temperature and filter through a special nitrogen protection device. The filter cake is red phosphorus. , put it in a special container and keep it airtight. The filtrate is distilled at atmospheric pressure through a fractionation column (equivalent to 5-15 theoretical plates), and the fraction at 80-82°C is collected to obtain a colorless or light yellow fuming liquid 85-98g, content 97-99% (silver nitrate titration), yield 82-94% (based on hexamethyldisiloxane), stored in a...
Embodiment 2
[0044] (1) Add 1,3-dimethylimidazolinone (industrial product, 176L), hexamethyldisiloxane (industrial product, 162kg, 1.0kmol) and zinc chloride (industrial product, 2.0kg) to In a 1000L glass-lined reaction kettle, at 25°C, add phosphorus tribromide (industrial product, 550kg, 2.03kmol) dropwise under stirring. After the drop is complete, continue to stir for 30 minutes, then raise the temperature to 95-105°C for 8 hours. Gas chromatographic analysis shows that the reaction of hexamethyldisiloxane has been completely completed. After the reaction is completed, it is cooled to ambient temperature and filtered through a special nitrogen protection device. The filter cake is red phosphorus, which is placed in a special container and sealed. Carry out atmospheric distillation through a rectification tower (equivalent to 5-15 theoretical plates), collect fractions at 80-82°C, and obtain 273-305 kg of colorless or light yellow fuming liquid, with a content of 99%-99.5% (Silver nitr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com