Solvent thermal constant-pressure synthesis method for controlling phase of carbon nitride material
A synthesis method and carbon nitride technology, applied in the fields of nitrogen and non-metallic compounds, nitrogen purification/separation, etc., can solve the problems of difficult preparation conditions, reduce the application value of products, damage the selectivity of the reaction process, etc., and achieve high application value. , to achieve the effect of low-cost large-scale synthesis and scale-up of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: firstly add metal sodium flakes into benzene, and distill after standing for 24 hours to remove water and oxygen therein. Then under the protection of nitrogen, weigh the stoichiometric ratio of sodium azide (NaN 3 ) and trichloroazine (C 3 N 3 Cl 3 ) into the autoclave, then add 8ml of dried benzene and then seal it. Apply a pressure of 40 MPa on the autoclave, then control the temperature of the autoclave to rise to 220 °C at a rate of 0.01 °C / min, react at constant temperature and pressure for 8 hours, and then naturally cool to room temperature.
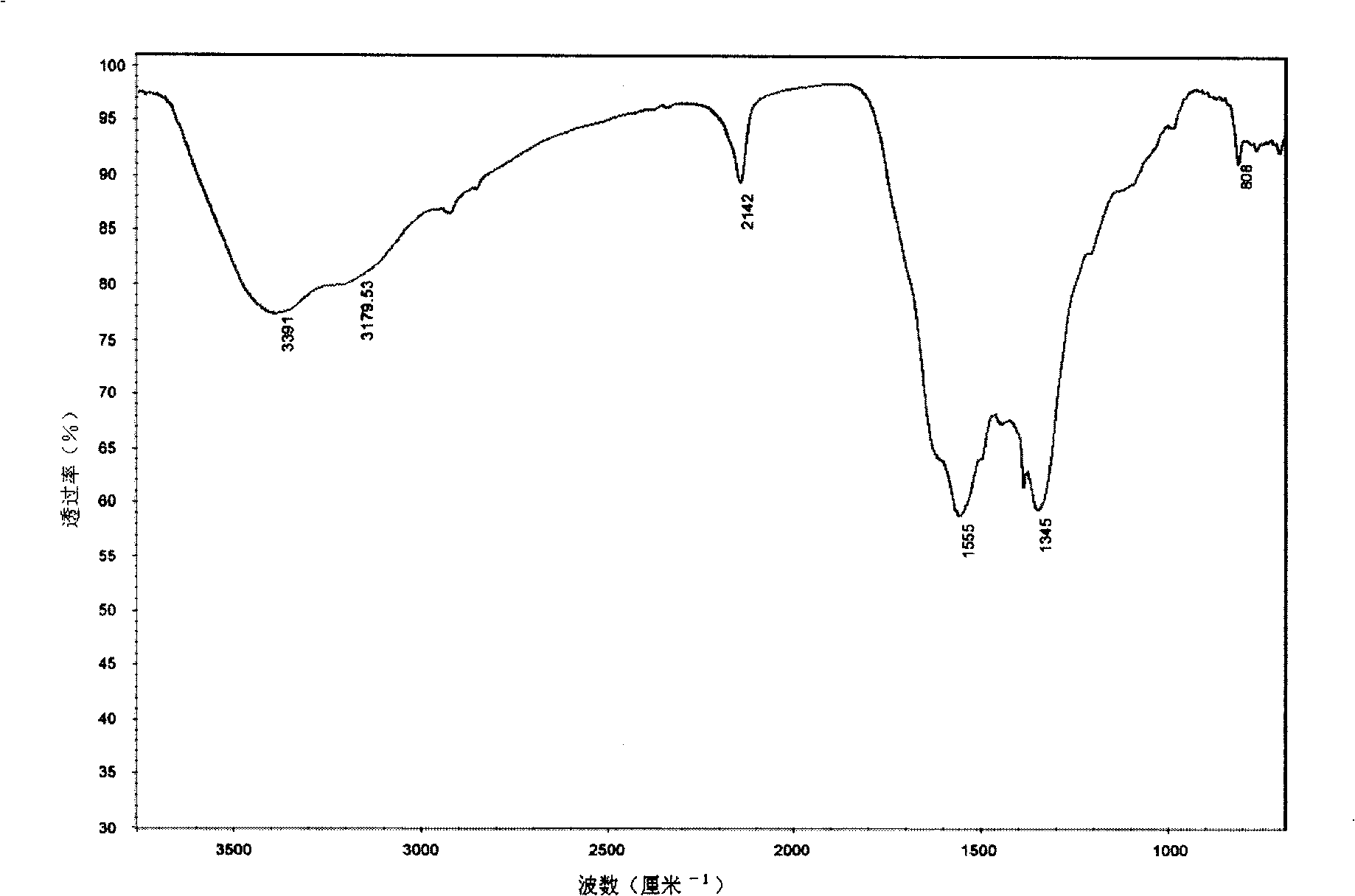
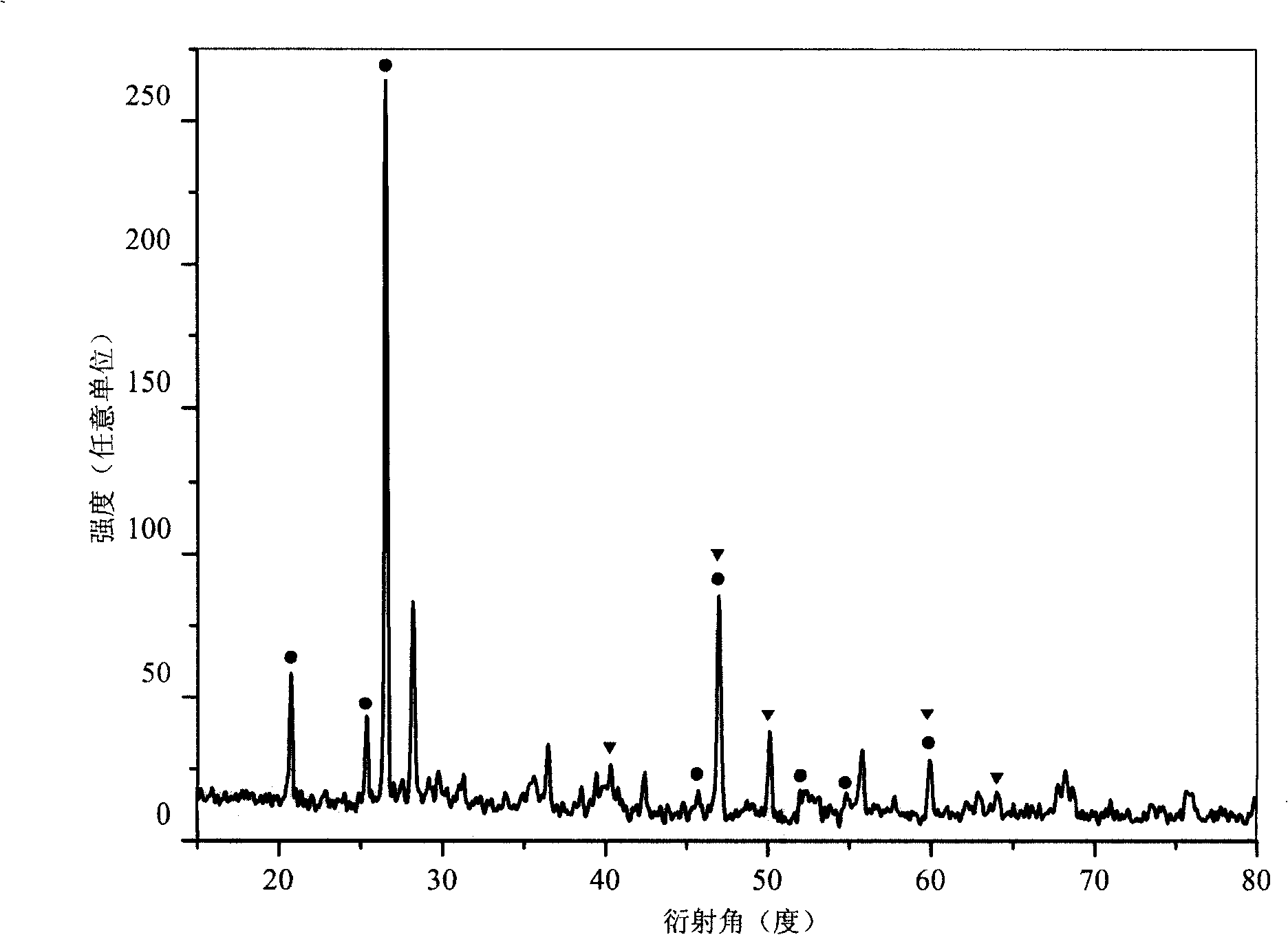
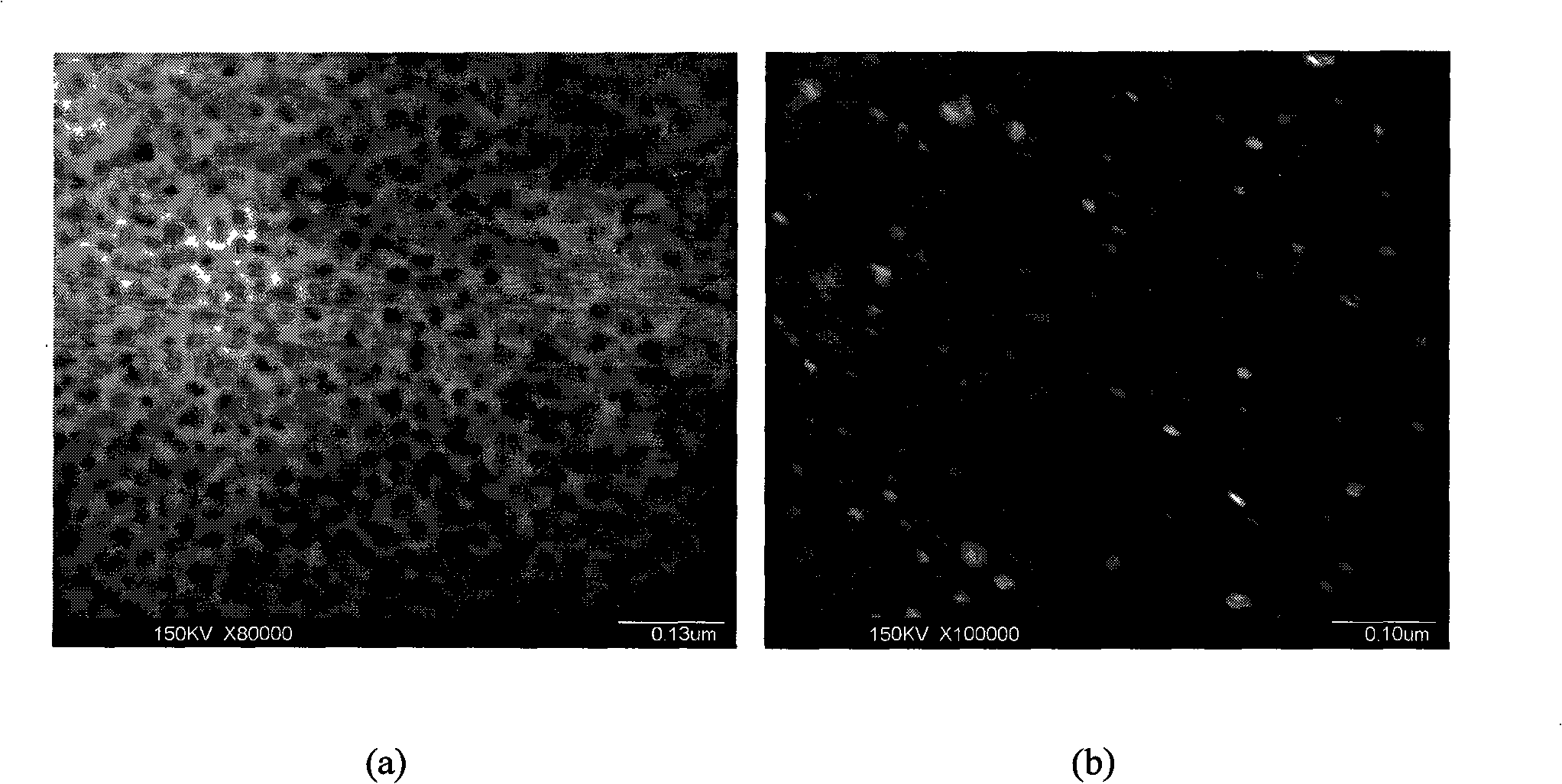
[0039] After the reaction, the benzene was first filtered off with suction, and then the product was washed with acetone, ethanol, and dilute hydrochloric acid in sequence to remove organic by-products and other impurities, and then the product was repeatedly washed with deionized water until the filtrate was neutral. Heating the product in vacuum to 60°C for drying can obtain graphite carbon nitride (g-C 3...
Embodiment 2
[0040] Example 2: As described in Example 1, the difference is that the reaction temperature is increased to 260°C.
Embodiment 3
[0041] Embodiment 3: As described in Embodiment 1, the difference is that the reaction temperature of the autoclave is increased to 300° C., trichloroazine is replaced by trimethylamine, and sodium azide is replaced by nitrogen trichloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com