Ortho-position imine ester or cyano substituted aryl thioether derivative, and preparation method and application thereof
A technology of cyano-substituted and aryl sulfides, which is applied in the field of preparation and application of ortho-imide esters or cyano-substituted aryl sulfide derivatives, and can solve the problems of poor synthetic regioselectivity, weak synthetic potential and difficult product Subsequent transformation and other issues, to achieve the effect of good functional group universality and efficient construction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0095] In the present invention, the preparation method preferably includes the following steps: in an air atmosphere, add imidate compound II (0.1 to 0.20 mmol), disulfide III (0.1 to 0.20 mmol), and trivalent rhodium in sequence in a 15 mL Schlenk reaction tube Catalyst [Cp*RhCl 2 ] 2 (0.6~2.4mg), bistrifluoromethanesulfonimide silver salt (2.3~6.9mg), sodium acetate (2.5~4.9mg), copper acetate or silver acetate (12.0~33.4mg), 1,2-di Ethyl chloride (DCE, 1mL), the end of the reaction was confirmed by thin-layer chromatography analysis, the reaction solution was filtered through diatomaceous earth and then concentrated into dry powder by rotary evaporation with 400 mesh silica gel, and then the reaction product was separated by column chromatography, 400 5 grams of mesh silica gel, the developer is petroleum ether and ethyl acetate with a volume ratio of 50:1 to 5:1, to obtain the thioether compound I of the ortho-imide or cyano group.
[0096] Wherein, imidate raw material...
Embodiment 1
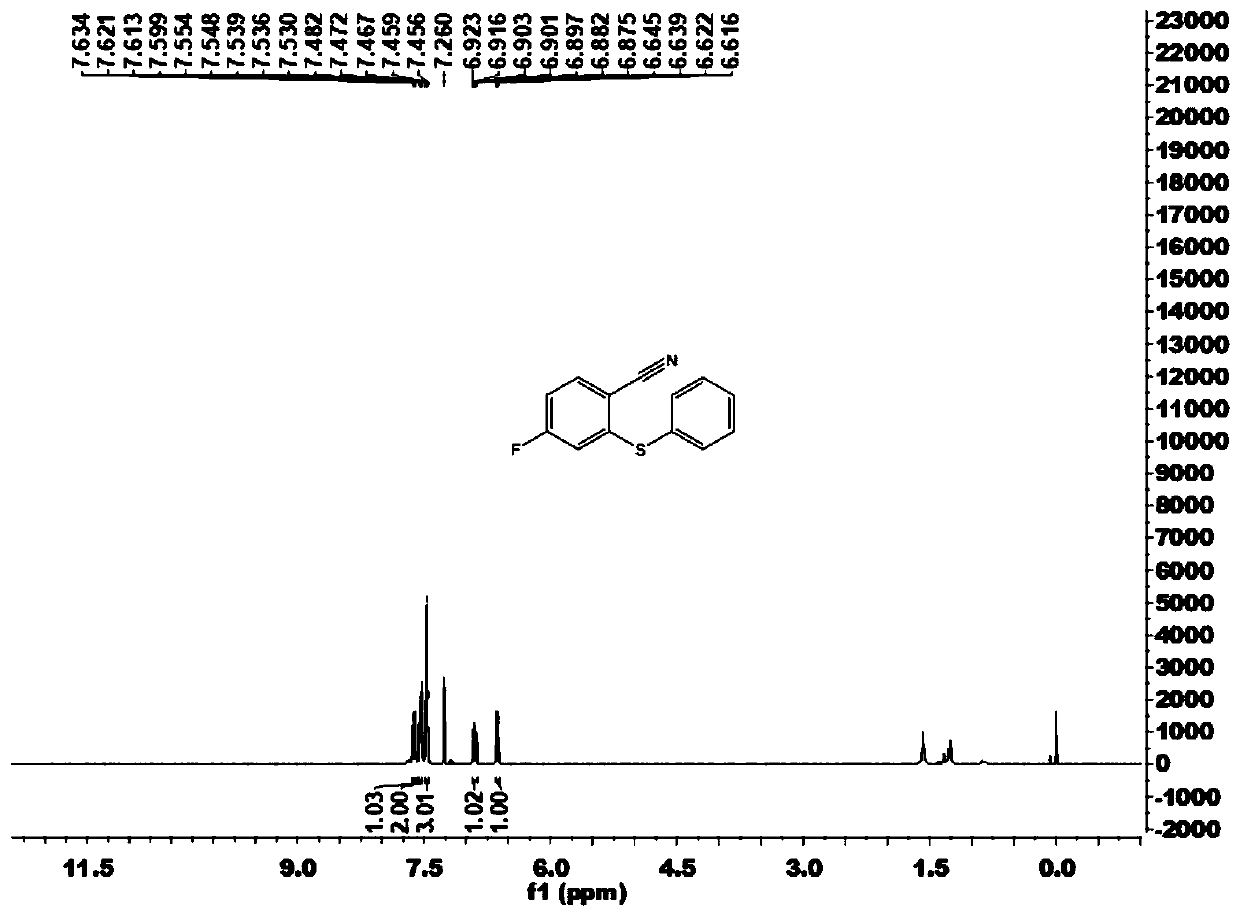
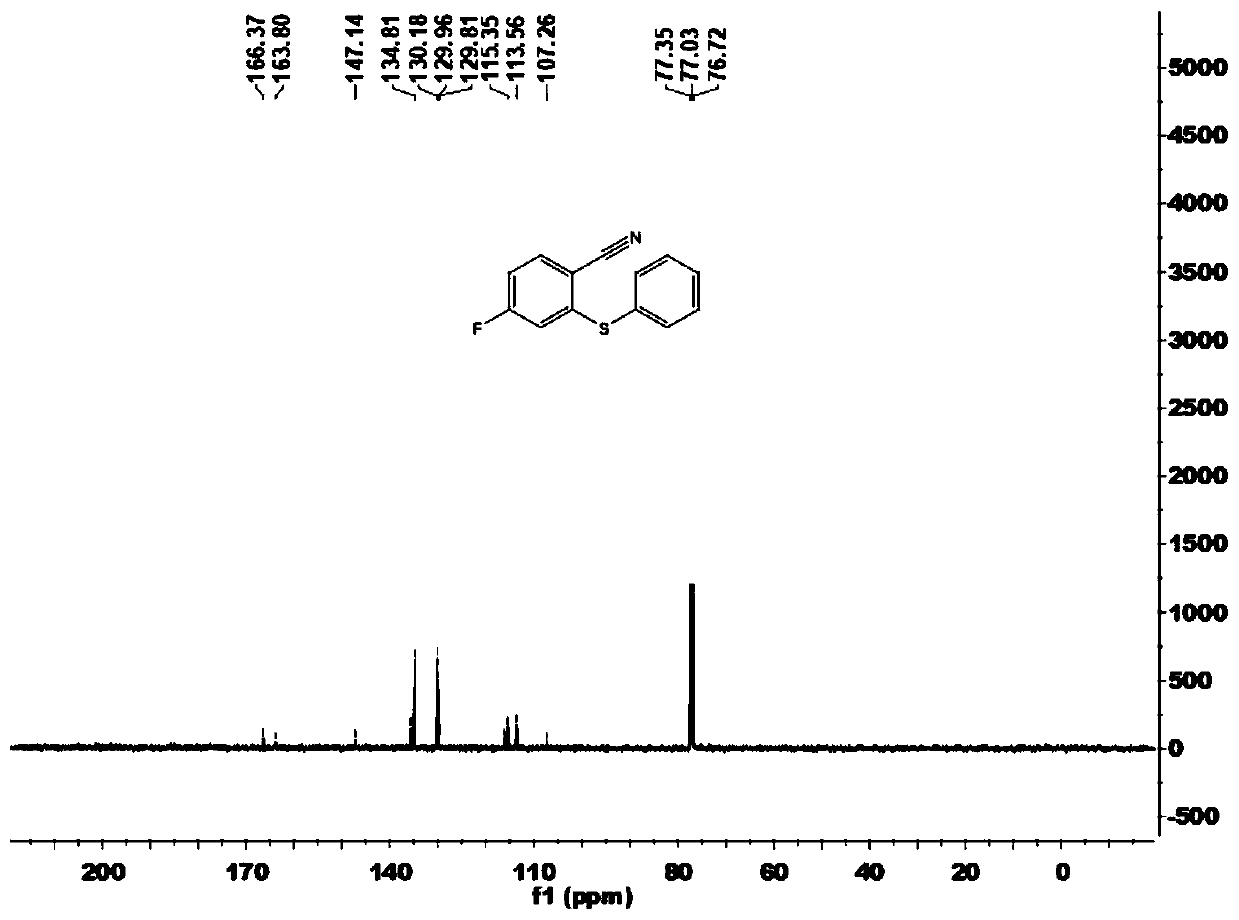
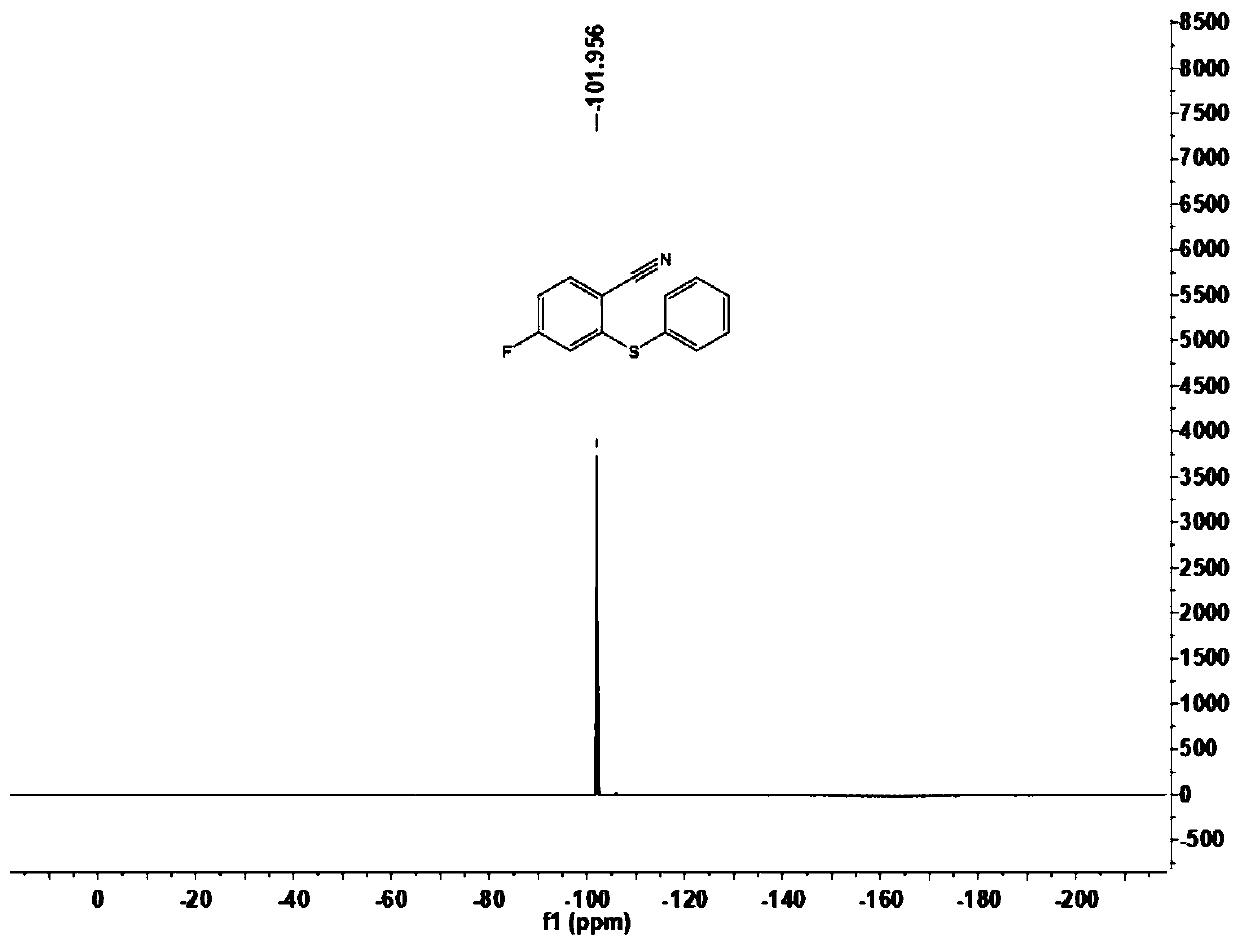
[0102] This embodiment carries out the preparation of 4-fluoro-2-(phenylthio)benzonitrile (1a), and its reaction formula is as follows:
[0103]
[0104] Under an atmosphere of atmospheric pressure air, add imidate compound 2a (33.4mg, 0.20mmol), disulfide 3a (43.6mg, 0.20mmol) successively to 15mL Schlenk reaction tube, trivalent rhodium catalyst [Cp*RhCl 2 ] 2 (1.2mg), bistrifluoromethanesulfonimide silver salt (2.3mg), sodium acetate (2.5mg), copper acetate (12.0mg), 1,2-dichloroethane (DCE, 1mL), at temperature It was reacted at 100°C for 12 hours. Cool to room temperature after completion of the reaction, filter through diatomaceous earth, and concentrate to obtain the crude product. The crude product was chromatographically separated on a prepared silica gel plate, and the selected developer or eluent was a volume ratio of petroleum ether to ethyl acetate of 50:1, and the product 4-fluoro-2-(phenylthio)benzene was isolated. Carbonitrile (1a), 35.7 mg, yield 78%.
...
Embodiment 2
[0108] The present embodiment carries out the preparation of 4-(chloromethylene)-2-(phenylthio)benzonitrile (1b), and its reaction formula is as follows:
[0109]
[0110] Under an atmosphere of atmospheric pressure air, add imidate compound 2b (39.2mg, 0.20mmol), disulfide 3a (43.6mg, 0.20mmol) successively to 15mL Schlenk reaction tube, trivalent rhodium catalyst [Cp*RhCl 2 ] 2 (1.2mg), bistrifluoromethanesulfonimide silver salt (4.6mg), sodium acetate (2.5mg), copper acetate (20.0mg), 1,2-dichloroethane (DCE, 1mL), at temperature It was reacted at 100°C for 12 hours. Cool to room temperature after completion of the reaction, filter through diatomaceous earth, and concentrate to obtain the crude product. The crude product was chromatographically separated on a prepared silica gel plate, and the selected developing solvent or eluent was a volume ratio of petroleum ether to ethyl acetate of 50:1, and the product 4-(chloromethylene)-2-( Phenylthio)benzonitrile (1b), 25.9 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com