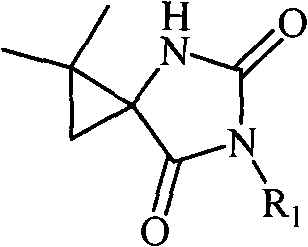
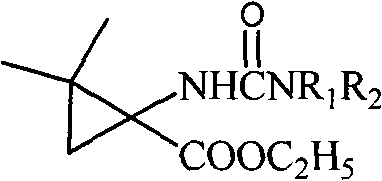
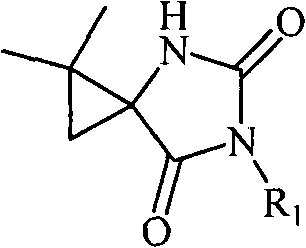
5-cyclopropane toroid hydantoin derivatives as well as preparation method and uses thereof
A technology of spirohydantoin and cyclopropane, which is applied in the field of 5-cyclopropane spirohydantoin derivatives and their preparation and application, can solve the problems of large side effects, gingival hyperplasia, etc., and achieve simple preparation method, The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: 1-isocyanate-2, the synthesis of ethyl 2-dimethylcyclopropanecarboxylate (compound 2)
[0023] Dissolve ethyl 1-carboxy-2,2-dimethylcyclopropanecarboxylate 1 (10mmol) in anhydrous tetrahydrofuran (30mL), cool in an ice-salt bath to below 0°C to -20°C, and then add chloroformic acid in sequence Ethyl ester and triethylamine or N-methylpyrrolidone (NMM), immediately produced a white precipitate. At this temperature, after stirring the mixture for 20 minutes, the NaN 3 5 mL of aqueous solution was added to the reaction solution, and stirring was continued for 1 hour. After the reaction was completed, a small amount of water was added to dissolve the insoluble matter, extracted with ethyl acetate, washed with saturated brine (2×10mL), washed with anhydrous Na 2 SO 4 Let dry overnight. After filtration, the solvent was evaporated under reduced pressure to obtain a light yellow liquid (note: compounds containing azide are explosive and cannot be evaporated t...
Embodiment 2
[0024] Example 2: Synthesis of 2,2-dimethyl-1-ureidocyclopropanecarboxylic acid ethyl ester (compound 3a)
[0025] At room temperature, all the colorless thick liquid (compound 2) obtained in Example 1 was dissolved in tetrahydrofuran (30 mL), 28%-30% ammonia water (about 8 mmol) was dissolved in a small amount of tetrahydrofuran, and then added to the above compound 2 in tetrahydrofuran solution, reacted for 15 minutes, evaporated the solvent under reduced pressure, and performed column chromatography immediately to obtain compound 3a. The product is a white solid; Yield: 52%; Melting point: 127-129°C; IR(KBr): 3355(N-H), 1704(C=O), 1658(C=O), 1534, 1365, 1318cm -1 ; 1 H NMR (300MHz, CDCl 3 ): δ0.98(d, 1H, J=4.6Hz, Cpr-H), 1.20(s, 3H, -CH 3 ), 1.27(t, 3H, J=7.2Hz, -CH 3 ), 1.29 (s, 3H, -CH 3 ), 1.72(d, 1H, J=4.6Hz, Cpr-H), 4.19(q, 2H, J=7.2Hz, CH 2 ), 4.97-5.01 (m, 2H, NH), 5.62 (br s, 1H, NH); MS (m / z): 223[M+Na]; Anal.Calcd.forC 9 h 16 N 2 o 3 : C, 53.98; H, 8.05;...
Embodiment 3
[0026] Example 3: Synthesis of ethyl 2,2-dimethyl-1-[3-(4-methoxycarbonylphenyl)ureido)cyclopropanecarboxylate (compound 31)
[0027] At room temperature, all the colorless thick liquid (compound 2) obtained in Example 2 was dissolved in tetrahydrofuran (30 mL), p-methoxycarbonylaniline (8 mmol) was dissolved in tetrahydrofuran, and then added to the THF solution of the above compound 2 , reacted for 30 minutes, evaporated the solvent under reduced pressure, and immediately column chromatographed to obtain compound 31. White solid; Yield: 45%; Melting point: 159-161°C; IR(KBr): 3391(N-H), 1712(C=O), 1687(C=O), 1608, 1589, 1525, 1438, 1384cm -1 ; 1 H NMR (300MHz, DMSO-d 6 ): δ1.04(d, 1H, J=5.1Hz, Cpr-H), 1.23(s, 3H, -CH 3 ), 1.27 (s, 3H, -CH 3 ), 1.26(s, 3H, -CH 3 ), 1.83(d, 1H, J=5.1Hz, Cpr-H), 3.86(s, 3H, CH 3 ), 4.18-4.25 (m, 2H, CH 2 ), 7.36(d, 2H, Ar-H), 7.85(d, 2H, Ar-H); 13 C NMR (150MHz, DMSO-d 6 ): δ14.94, 20.27, 22.67, 27.28, 29.11, 42.86, 52.43, 61.13, 117.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com