Medicine use of beta-methoxy acrylic ester compounds as novel STAT3 restrainer
A compound, the technology of isopropoxy, which is applied in the field of β-methoxyacrylate, can solve the problems that there are no STAT3 inhibitors of β-methoxyacrylate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
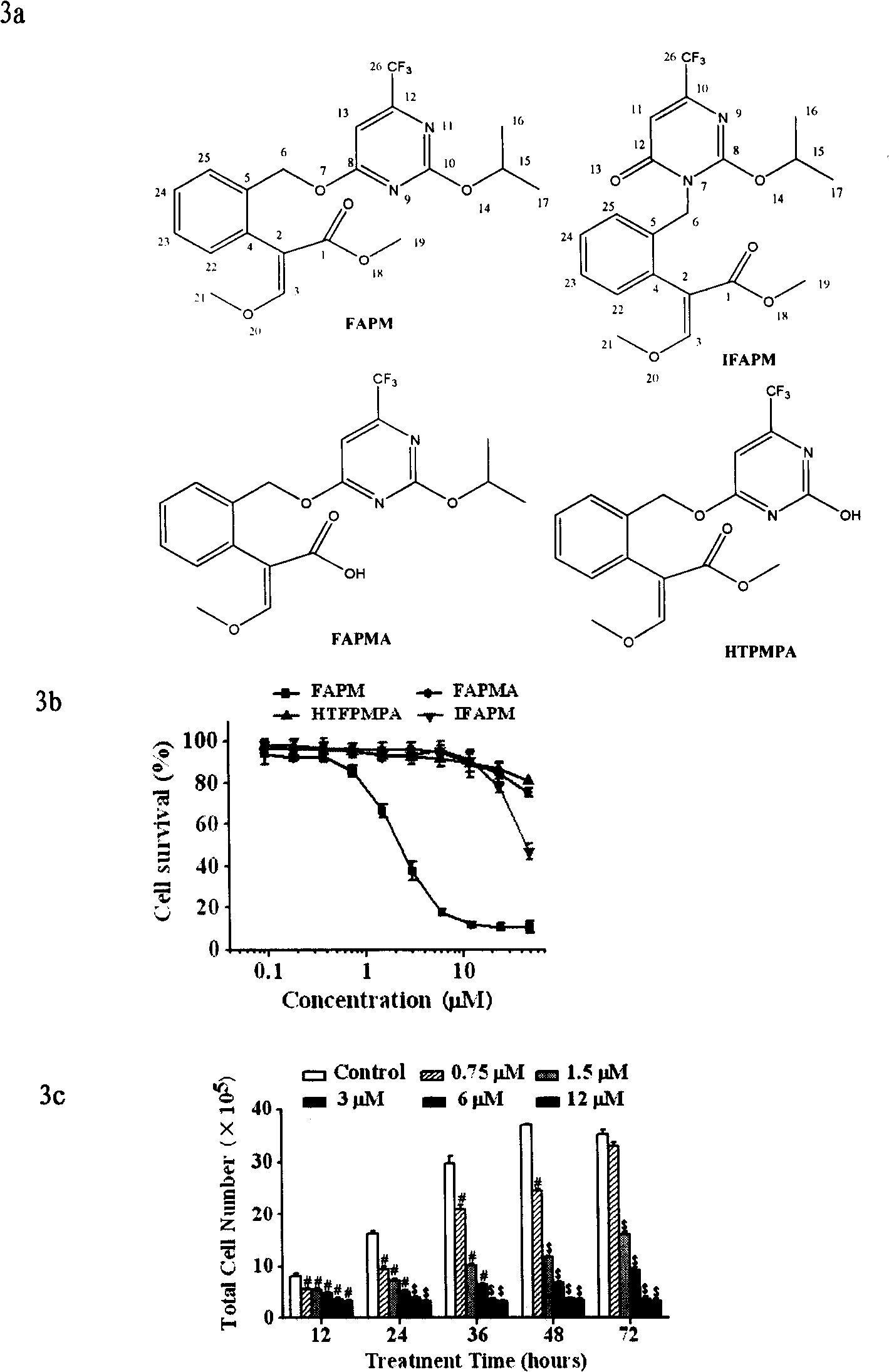
[0033] Example 1 (E)-3-methoxy-2-[2-(2-isopropoxy-6-trifluoromethylpyrimidine-4-oxyl group) phenyl] methyl acrylate (compound 1, Fluacrypyrim , FAPM) preparation
[0034] (1) Methyl o-tolylacetic acid: Weigh 7.5g (0.05mol) o-tolylacetic acid, dissolve it in 75mL of anhydrous methanol, add 0.1mL of sulfuric acid under stirring, heat and reflux for 2 hours, then cool and depressurize Recover the solvent, add 30mL of dichloromethane and 20mL of water, separate the layers, extract the water layer once with 15mL of dichloromethane, combine the dichloromethane solution, wash with water once, dry over anhydrous sodium sulfate, filter off the solid, and recover the solvent. A colorless liquid was obtained. It can be directly used in the next reaction.
[0035] (2) 3-Hydroxy-2-(2-methylphenyl)-2-methyl acrylate: Weigh 2.50g (0.062mol) of sodium hydride, suspend in 25mL of dry tetrahydrofuran, add under ice water cooling and stirring 20 mL of methyl formate dried over anhydrous sodiu...
experiment example 2
[0041] Experimental example 2 (E)-3-methoxy-2-[2-(3,4-dihydro-2-isopropoxy-4-oxo-6-trifluoromethylpyrimidin-1-yl)benzene base] the preparation of methyl acrylate (compound 2, IFAPM)
[0042] (1)-(6) steps are the same as the preparation of compound 1, and the operation of step (7) is basically the same as that of compound 1, except that the target product to be separated is the second (rear) main point after column chromatography separates compound 1, Yield 5.85g, mp 94-96°C. 1H-NMR (CDCl3, ppm) δ: 1.089 (br-s, 6H), 3.621 (s, 3H), 3.843 (s, 3H), 4.90 (br-s, 1H), 5.231 (m, 2H), 6.457 (s, 1H), 7.09-7.15 (m, 2H), 7.24-7.29 (m, 2H), 7.484 (s, 1H).
[0043] The following biological activity experiments are used to further illustrate the present invention.
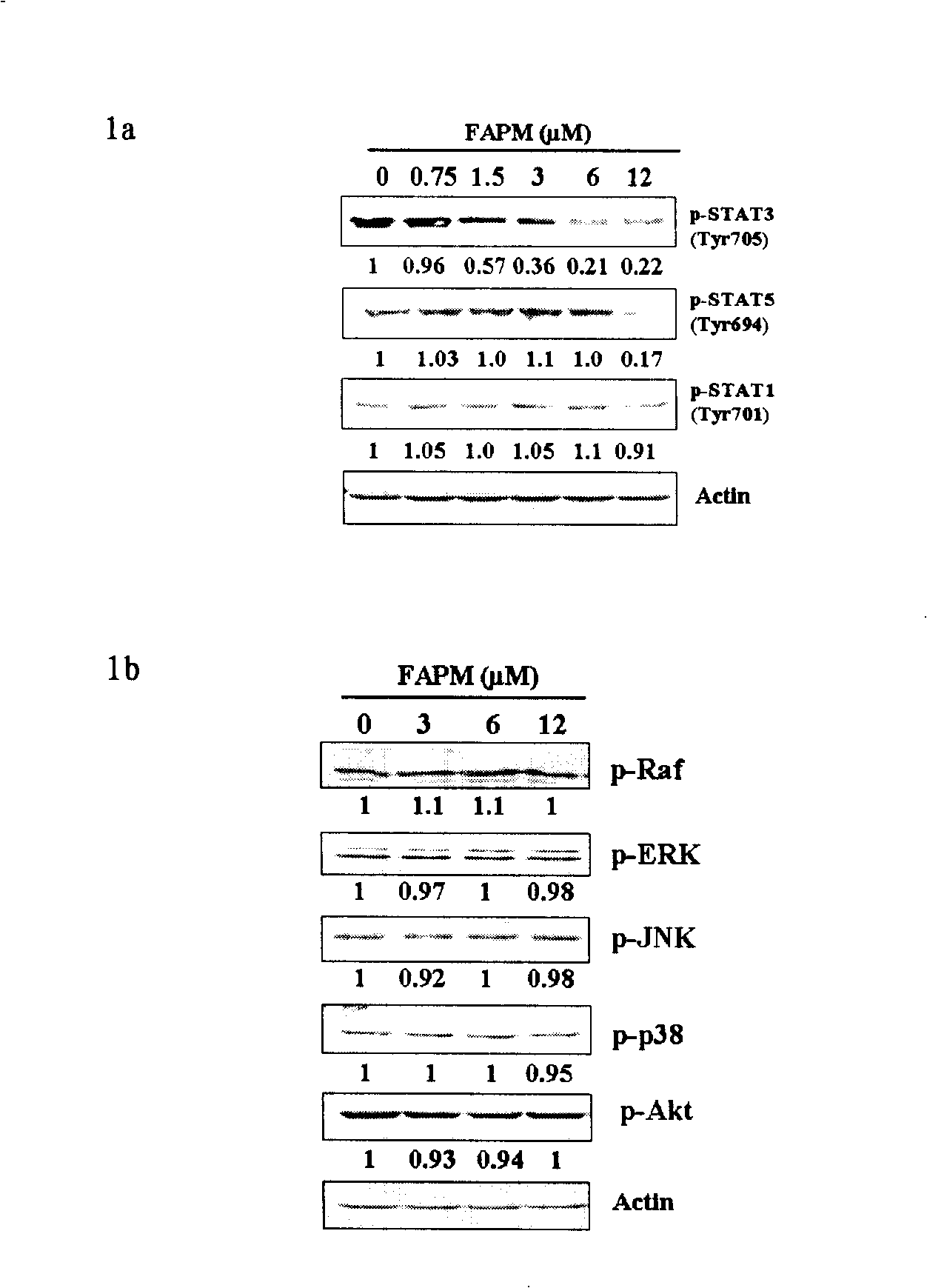
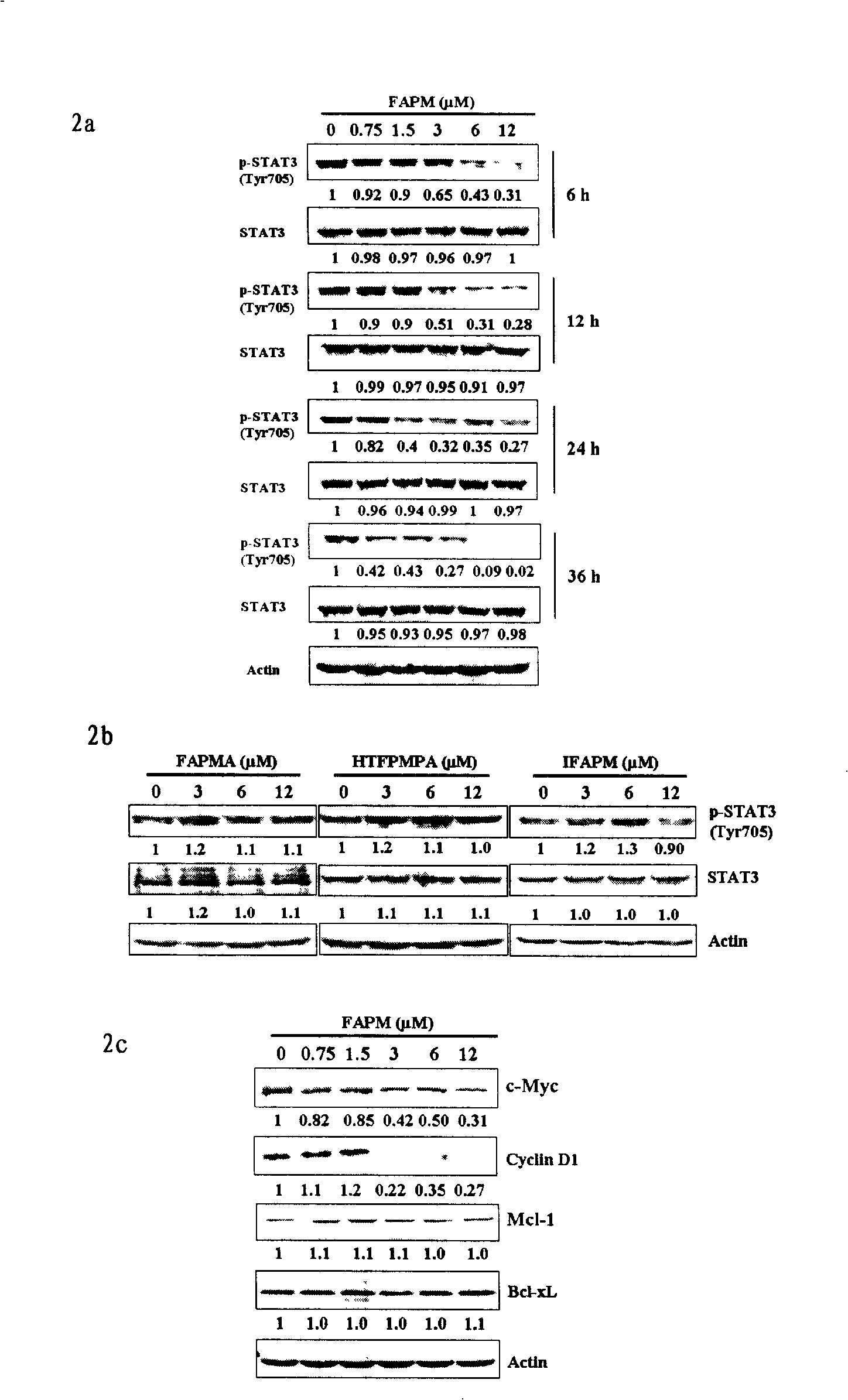
[0044] Biological effect experiment 1 Effect of FAPM on the expression levels of STAT family, PI3 / Akt and MAPK signaling pathway signaling molecules in cells
[0045] figure 1 Shown is the effect of FAPM on the expression l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com