Transgenic cell overlapped vascular inner rack and manufacture method thereof
A technology of transgenic cells and internal stents, applied in the field of intravascular stents, can solve the problems that cannot meet the requirements of planting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] Below in conjunction with accompanying drawing, the present invention will be further described, but therefore the present invention is not limited among the scope of described embodiment:
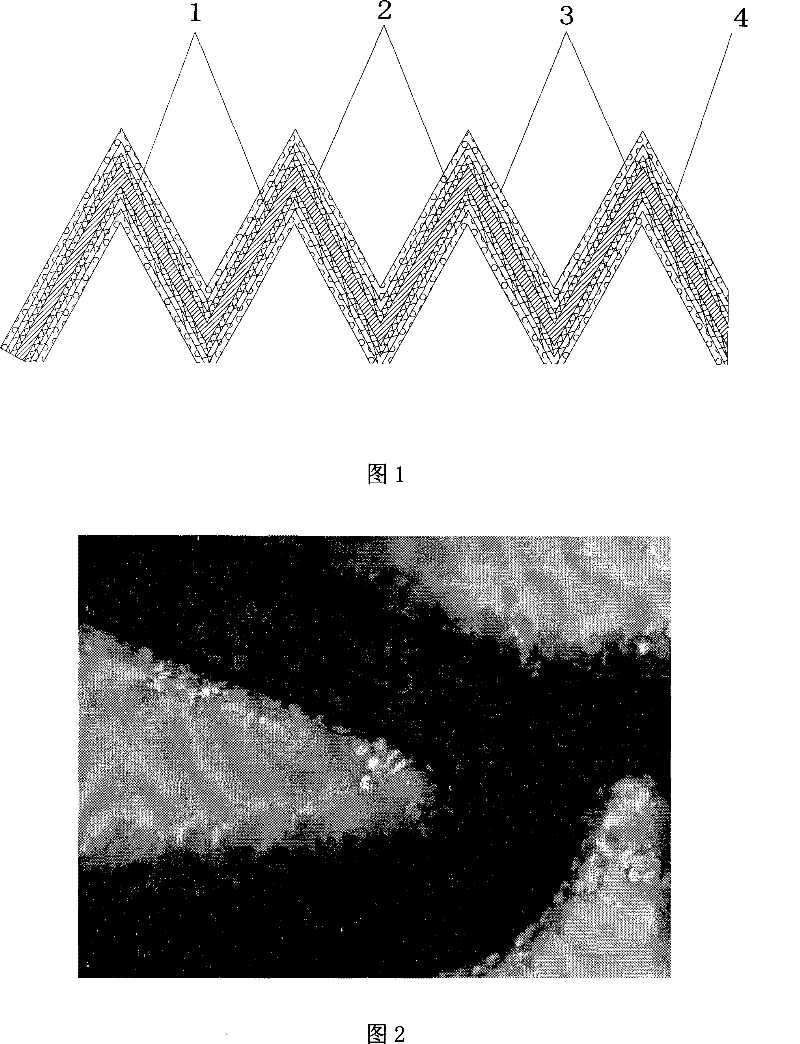
[0036] see figure 1: The intravascular stent covered by the transgenic cells is provided with a treatment layer 1 on the metal surface of the traditional bare metal stent 4, an adhesion matrix coating 2 is arranged on the treatment layer 1, and a cell coating 3 is established on the adhesion matrix coating , Cell Coating 3 was obtained by stably transfecting cells with the gene of interest.
[0037] Wherein, the treatment layer 1 is a low-temperature plasma coating, and its surface roughness is 5-15 μm. Adhesion matrix coating 2 is one of protein coating and sequence peptide coating. The protein is one or more of proteins with the function of promoting cell adhesion, such as gelatin, type IV collagen, polylysine, and fibronectin. Sequence peptides are sequence peptides with the f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface roughness | aaaaa | aaaaa |
| surface roughness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com