Method for preparing stoichiometric proportion lithium niobate or lithium tantalate wafer
A stoichiometric ratio, lithium niobate technology, applied in chemical instruments and methods, single crystal growth, crystal growth, etc., can solve the problems of low yield, uneven distribution of components, and uncontrollable lithium content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
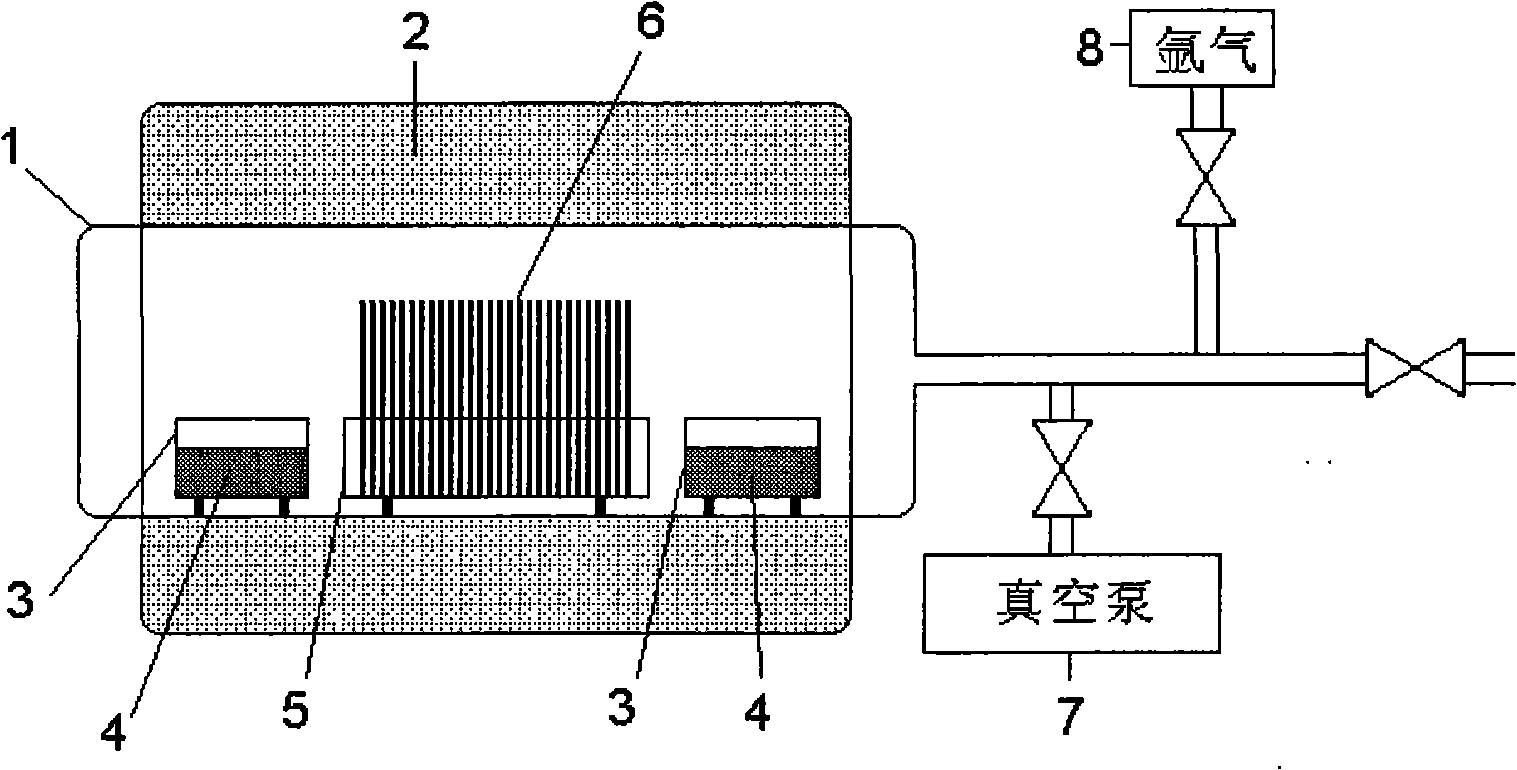
[0026] Technical scheme of the present invention constitutes as figure 1 As shown, in the airtight container 1, pack 5 pieces of lithium niobate wafers 6 placed on the wafer holder 5, the thickness of the wafer 6 is 0.6mm, and the diameter is 78mm. Lithium serves as the evaporation source container 3 of the evaporation source 4 .
[0027] First turn on the vacuum pump, vacuumize the inside of the airtight container 1 to make the vacuum less than 0.5 Pa, then turn off the vacuum pump, utilize the heating body 2 to increase the temperature in the airtight container 1, the temperature is 1100 degrees Celsius; keep at this high temperature for 75 hours, Then cool down to room temperature, open the airtight container 1, and the required stoichiometric lithium niobate wafer can be produced. The detected lithium molar content of the wafer was 49.9mol%±0.1.
Embodiment 2
[0029] In the airtight container 1, pack 5 lithium niobate wafers 6 placed on the wafer holder 5, the thickness of the wafer 6 is 1.2mm, and the diameter is 78mm. Evaporation source container 3 for source 4.
[0030] After evacuating the inside of the airtight container 1 and raising the temperature, it is kept at 1100 degrees Celsius for 100 hours, and then lithium niobate wafers with the required stoichiometric ratio can be produced. The detected lithium molar content of the wafer was 49.9mol%±0.1. Others are with embodiment 1.
Embodiment 3
[0032] In airtight container 1, put into 5 slices of lithium niobate wafers 6 placed on wafer support 5, the thickness of wafer 6 is 0.8mm, and diameter is 76mm, puts into full bloom 30g lithium as evaporation source simultaneously on both sides of wafer 4 of the evaporation source container 3 .
[0033] Vacuumize the inside of the airtight container 1 to make the vacuum less than 0.5 Pa, then turn off the vacuum pump, fill in high-purity argon, make the air pressure in the container 1 about 1000 Pa, and keep it at 1100 degrees Celsius for 80 hours to obtain the required Stoichiometric lithium niobate wafers. The detected lithium molar content of the wafer was 49.8mol%±0.1. Others are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com