Indirect sodium borohydride-hydrazine mixed fuel cell
A sodium borohydride, fuel cell technology, applied in indirect fuel cells, fuel cells, solid electrolyte fuel cells, etc., to improve stability, improve work efficiency, and improve the effect of hydrogen production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] There are three main methods for the preparation of tubular reactors, with Ni 3 Al and Co3 Al as a catalyst precursor material as an example:
[0031] method one:
[0032] Include the following steps:
[0033] (1) The catalyst precursor material Ni 3 Al and Co 3 Al powder is mixed according to the mass ratio of 0-100:100-0, filled into the metal support body, and sintered under the protection of vacuum or inert gas for 0.5-2 hours, then cooled to room temperature, and the sintering temperature is 550-700 °C; the catalyst precursor material The mass ratio to the metal support is 5-30:70;
[0034] (2) immerse the sintered metal support body in a sodium hydroxide or potassium hydroxide solution with a mass concentration of 5 to 20%, and carry out dealumination reaction at room temperature until bubbles no longer emerge. A metal nickel or metal cobalt catalytic layer with extremely strong catalytic activity is formed on the surface and inner surface;
[0035] (3) Fill...
Embodiment 1
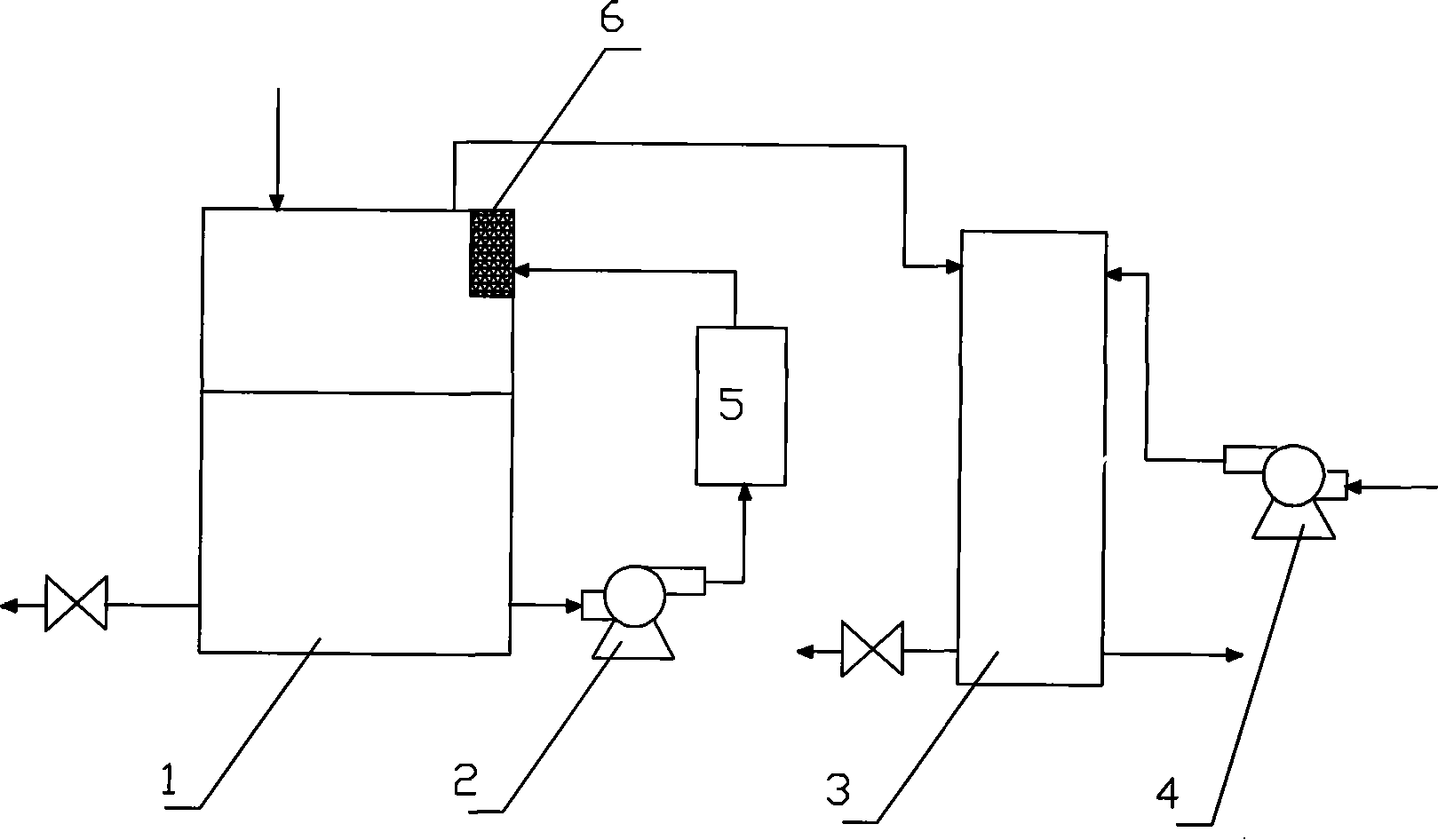
[0057] Embodiment 1: Fuel cell system composed of proton exchange membrane fuel cell
[0058] The sodium borohydride-hydrazine mixed solution fuel cell system consists of a hydrogen generator, a proton exchange membrane fuel cell 3 and a blower 4 . The hydrogen generator consists of a fuel tank 1 , a tubular reactor 5 and a filter 6 . The fuel tank 1 is provided with a feed inlet, a waste discharge outlet and an outlet of the sodium borohydride-hydrazine mixed solution leading to the infusion pump 2 (such as figure 1 shown). The fuel tank 1 and the tubular reactor 5 are connected by an infusion pump 2 . The tubular reactor 5 is provided with an inlet of the sodium borohydride-hydrazine mixed solution from the fuel tank 1 and an outlet of the hydrogen generation product. An integrated porous catalyst is housed in the tubular reactor 5 . The speed of the hydrolysis reaction of the sodium borohydride-hydrazine mixed solution is controlled by controlling the fuel flow.
[005...
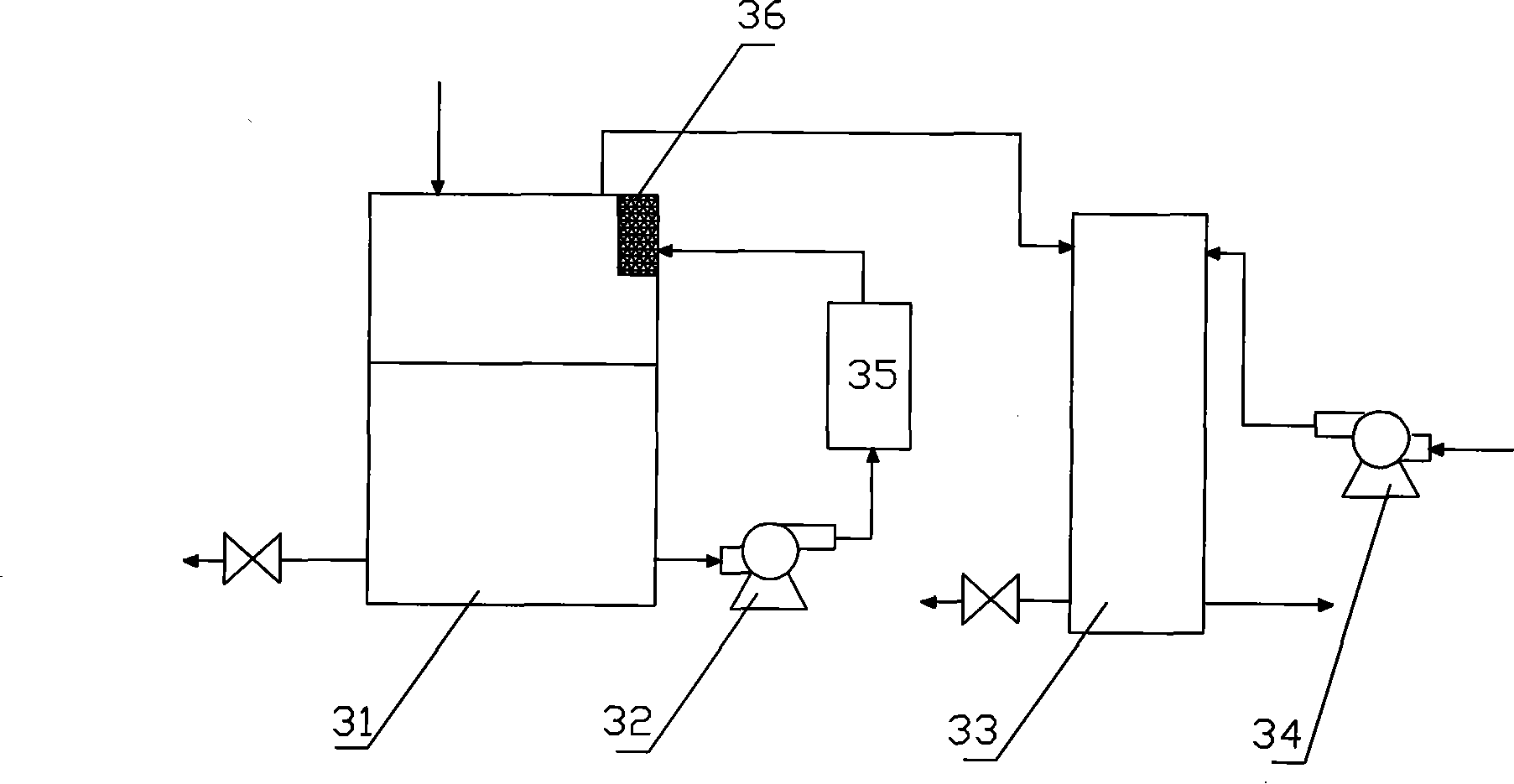
Embodiment 2
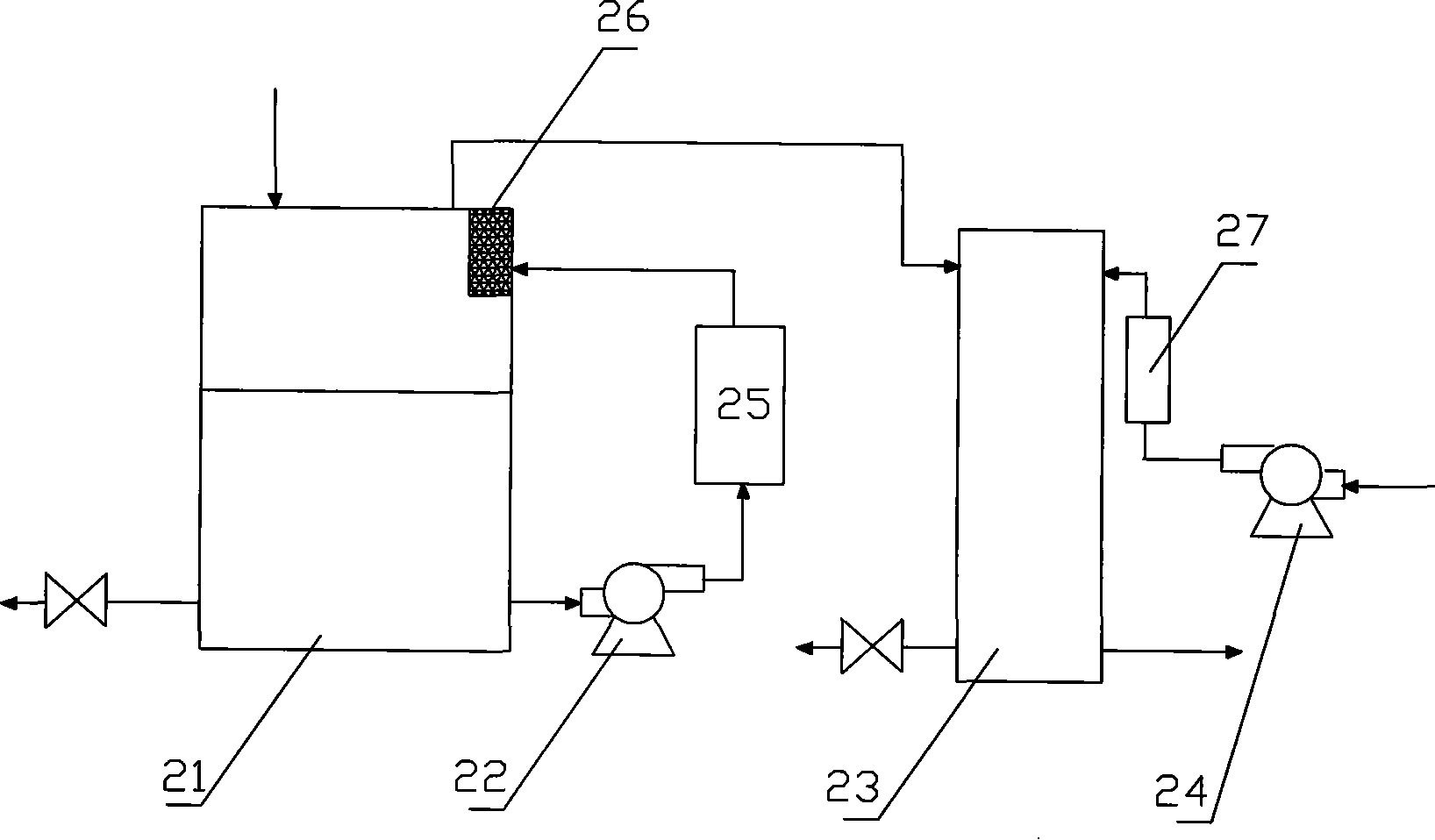
[0061] Example 2: Fuel cell system composed of alkaline fuel cell
[0062] The sodium borohydride-hydrazine mixed solution fuel cell system consists of a hydrogen generator, an anion exchange membrane fuel cell 23 and a blower 24 . The hydrogen generator consists of a fuel tank 21 , a tubular reactor 25 and a filter 26 . Same as embodiment 1, fuel tank 21 is provided with feed inlet, waste material discharge port and the sodium borohydride-hydrazine mixed solution outlet leading to infusion pump 22 (as figure 2 shown). The fuel tank 21 and the tubular reactor 25 are connected by an infusion pump 22 . The tubular reactor 25 is provided with an inlet of the sodium borohydride-hydrazine mixed solution from the fuel tank 21 and an outlet of the hydrogen generation product. The tubular reactor 25 is equipped with an integrated porous catalyst. The speed of the hydrolysis reaction of the sodium borohydride-hydrazine mixed solution is controlled by controlling the fuel flow.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| voidage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com