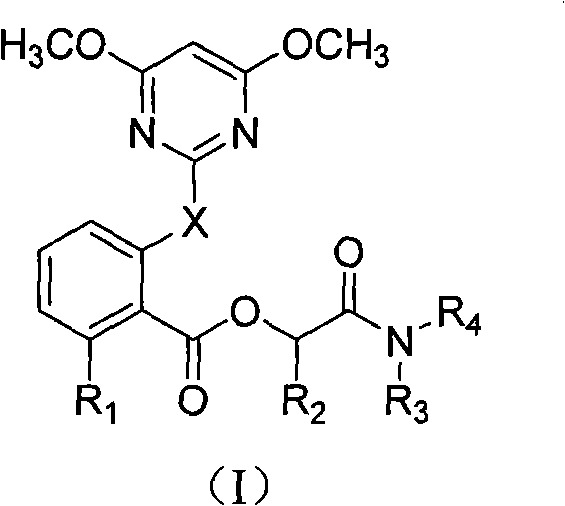
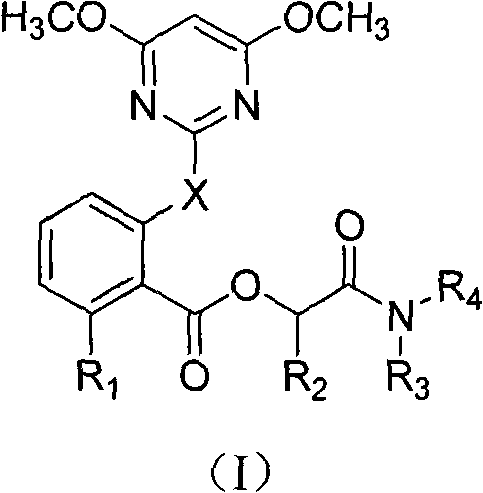
2-pyrimindinyloxy (pyrimindinylthio) benzoxy acetamide compound and application thereof
A technology of acetamide and benzoic acid, which is applied in the field of 2-pyrimidinyloxybenzoic acid acetamide compounds, and can solve the problems that do not involve 2-pyrimidinyloxy (thio) benzoic acid acetamide compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
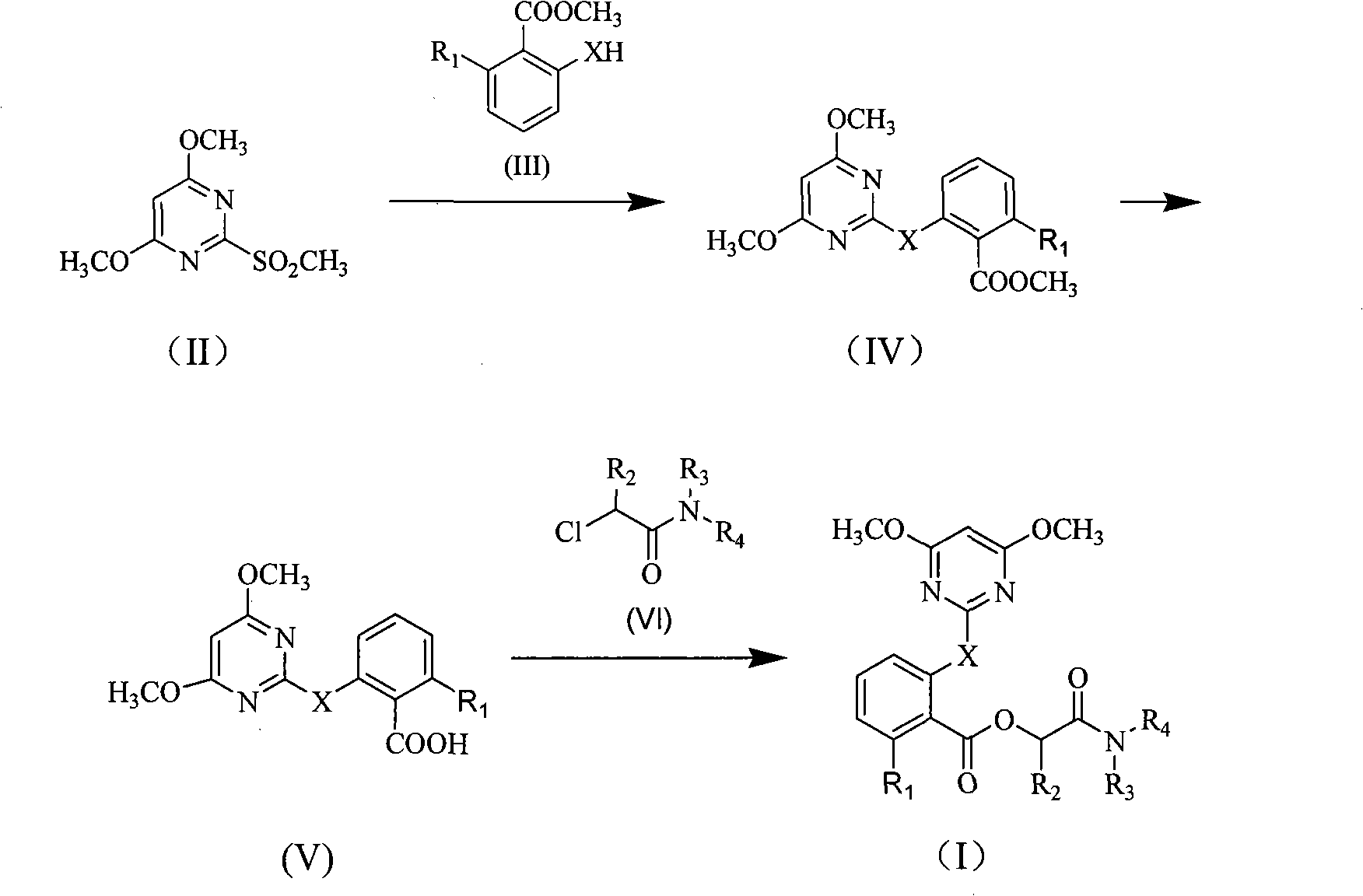
Synthetic example
[0106] Synthesis of compound 1:
[0107]
[0108] Add 4,6-dimethoxy-2-methanesulfonyl pyrimidine 7 grams (0.036 moles), methyl salicylate 6 milliliters (0.047 moles), potassium carbonate 6 grams (0.043 mol), N, N-dimethylformamide 100 ml. After stirring and reacting at 110-130°C for 4 hours, it was cooled to room temperature, and 200 ml of ethyl acetate and 180 ml of water were added to the reaction solution for extraction. The organic phase was washed successively with 50 milliliters of 1% dilute hydrochloric acid, 50 milliliters of saturated sodium bicarbonate, 3 × 50 milliliters of saturated aqueous sodium chloride, dried over anhydrous magnesium sulfate, and the residue obtained after precipitation was subjected to silica gel column chromatography (petroleum ether / ethyl acetate=5 / 1) to obtain 8.35 g of methyl 2-(4,6-dimethoxy-2-pyrimidinyloxy)benzoate.
[0109]
[0110] Add 2.93 grams (0.01 moles) of 2-(4,6-dimethoxy-2-pyrimidinyloxy) methyl benzoate, 50 millilite...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com