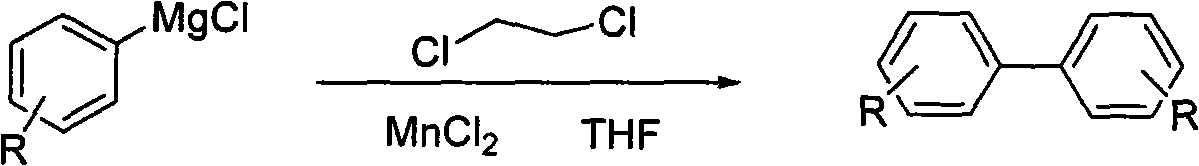
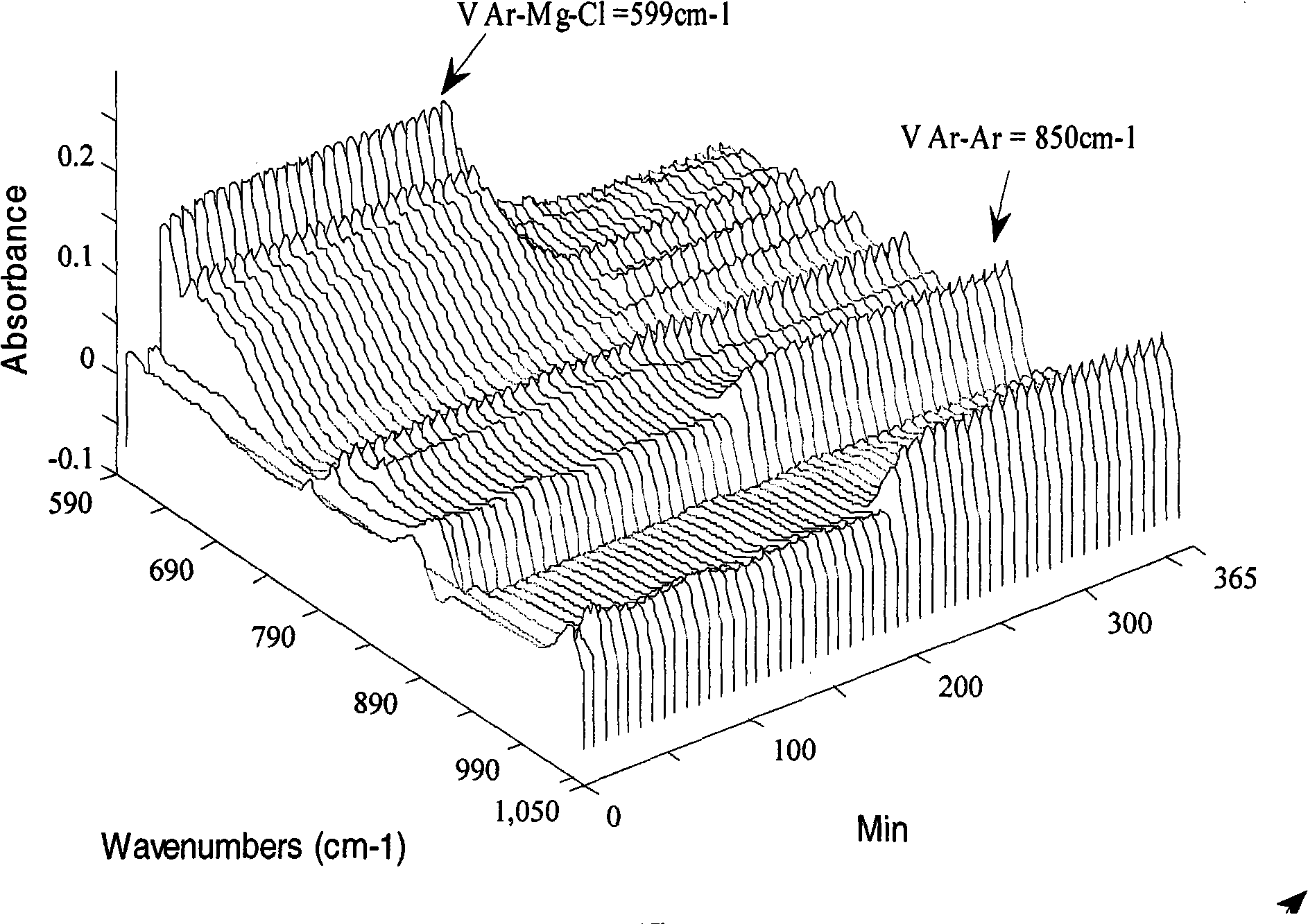
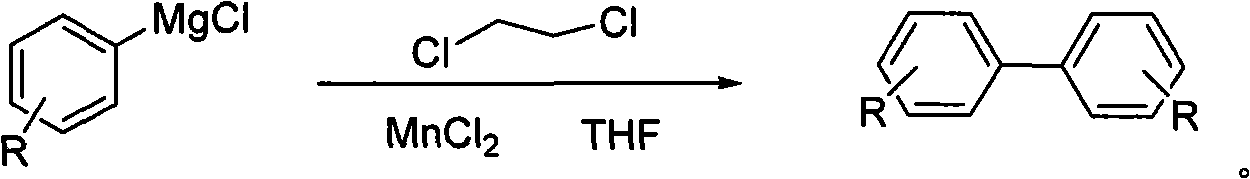
Self-coupling reaction method of manganses-catalyzed and oxidized chlorobenzene grignard reagent
A technology of aryl Grignard reagent and coupling reaction, which is applied in the field of synthesis of biaryl compounds, can solve the problems of non-negligible price of brominated aromatic hydrocarbons, shorten the reaction time, reduce the cost, etc., and achieve good catalytic effect and high reaction yield Good, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 30ml of anhydrous tetrahydrofuran (THF) solution of p-chlorotoluene Grignard reagent (which contains 9.7mmol p-chlorotoluene Grignard reagent) into a 50ml three-necked flask, and then add MnCl 2 (126mg, 1mmol), and 1,2-dichloroethane (1.19g, 12mmol) was slowly added dropwise with a syringe, and the addition was completed in 10 minutes. During the addition, nitrogen was protected, and the addition temperature was kept at 25°C. The reaction was stirred for 1 h, the reaction mixture was quenched with 2 ml of methanol, the reaction solution was filtered, and the filter cake was washed with tetrahydrofuran (THF), the filtrates were combined, and the tetrahydrofuran (THF) was evaporated under reduced pressure to obtain 900 mg of 4,4-dimethylbiphenyl crude product , the crude product was purified by silica gel column chromatography (the mobile phase volume ratio was n-hexane:ethyl acetate=50:1), and 850 mg (99% yield) of a white solid was obtained, which was 4,4'-dimethylbi...
Embodiment 2
[0024] The conditions are the same as in Example 1, except that the addition of p-chlorotoluene Grignard reagent is changed to the addition of o-chlorotoluene Grignard reagent, and the reaction result is that the corresponding biaryl compound yield is 87.4%.
Embodiment 3
[0026] The conditions are the same as in Example 1, except that the addition of p-chlorotoluene Grignard reagent is changed to p-methoxychlorobenzene Grignard reagent, and the reaction result is that the yield of the corresponding biaryl compound is 80.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com