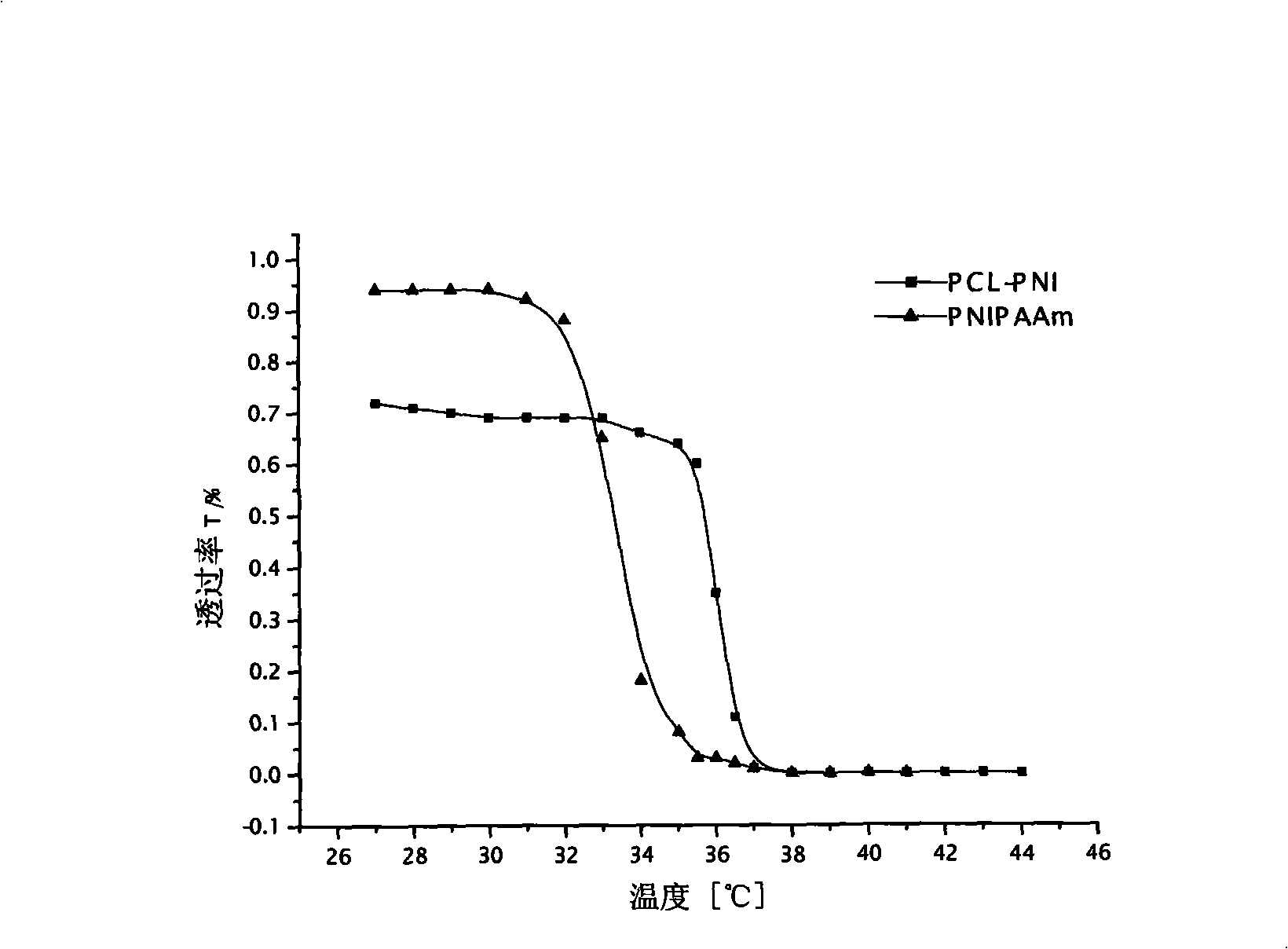
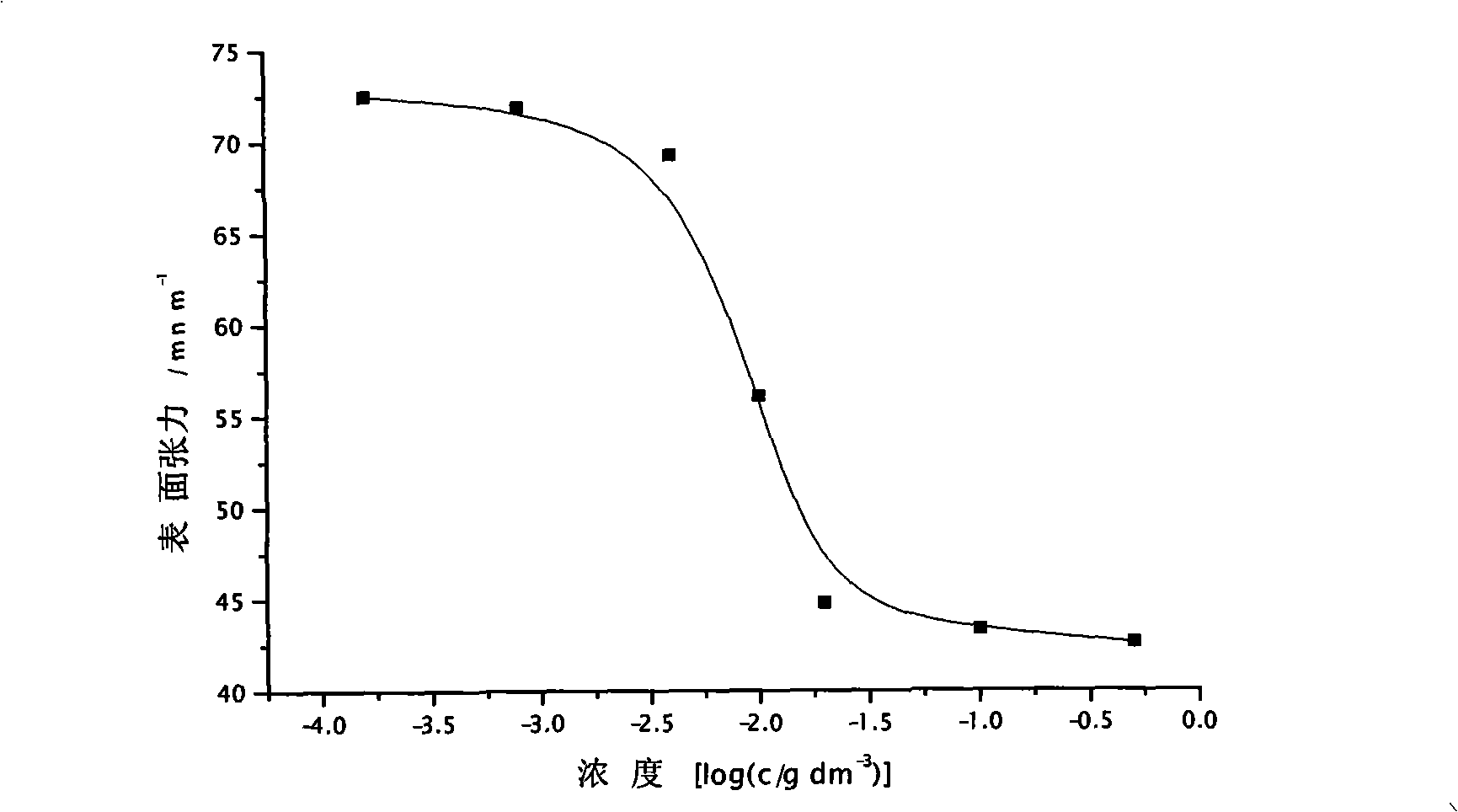
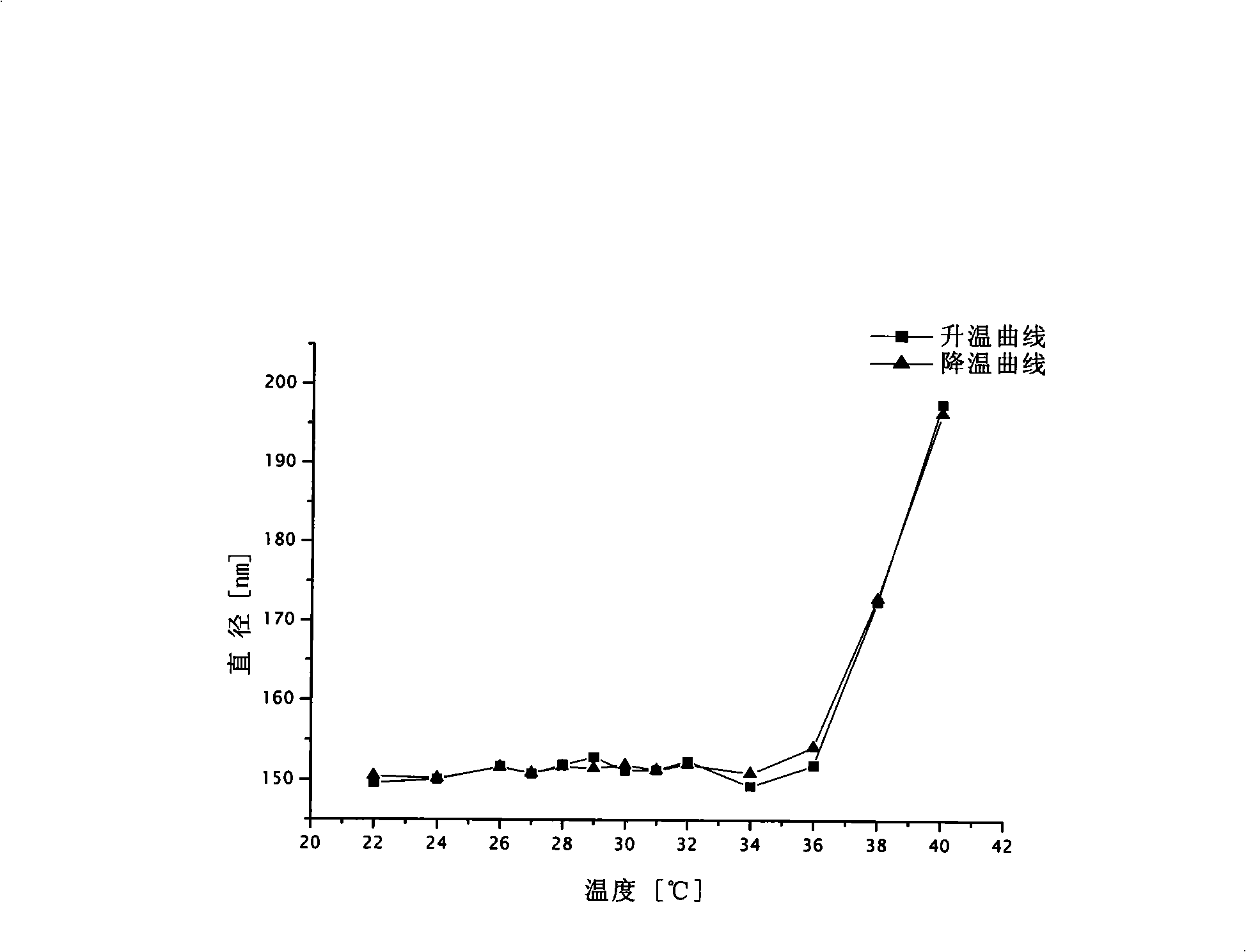
Polycaprolactone/polyacrylamide graft copolymer and uses thereof
A technology of graft copolymer and alkylacrylamide, which is applied in the field of polyε-caprolactone/polyN-alkylacrylamide graft copolymer, can solve the problems that have not been reported in the research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Preparation of carboxyl-terminated polymer N-isopropylacrylamide (PNIPAAm-COOH):
[0038] N-isopropylacrylamide (200mmol), 3-mercaptopropionic acid (4mmol) and azobisisobutyronitrile (4mmol) were dissolved in 70ml tetrahydrofuran. in N 2 In the atmosphere, react at 70°C for 24h, carboxyl-terminated polymer N-isopropylacrylamide (PNIPAAm-COOH). 1 H-NMR (CDCl 3 , δppm) characteristic peaks: 3.92 (s, H, NCH), 1.19 (s, 6H, CH 3 ). polymer molecular weight 1 The number average molecular weight calculated by H-NMR is: 5680.
[0039]
[0040] (2) Preparation of 4-carbonyl-ε-caprolactone and ε-caprolactone copolymer (PCL-OPD):
[0041] (2-1) Preparation of 4-carbonyl-ε-caprolactone:
[0042] 1,4-cyclohexanedione (0.1 mol) and m-chloroperoxybenzoic acid (0.12 mol) were reacted under reflux at 40° C. for 3 h in 200 ml of dichloromethane. Dichloromethane was distilled off under reduced pressure, and 80 ml of anhydrous diethyl ether was washed twice to obtain a white p...
Embodiment 2~3
[0060] The specific steps and operations of Examples 2 to 3 are basically the same as those in Example 1, except that the molar ratio of the chain transfer reagent 3-mercaptopropionic acid to N-isopropylacrylamide (NIPAAm) is changed to obtain endogenous chains with different molecular weights. carboxyl polymer. The specific data are shown in Table 1 below:
[0061] Table 1
[0062]
Embodiment 4~9
[0064] Embodiment 4~9 concrete steps and operation are basically the same as embodiment 1, and difference is by changing monomer / catalyst charge ratio (M / S n ), reaction time (T), monomer feed ratio (OPD / ε-caprolactone, f OPD ) and other conditions, the polymer composition (OPD / ε-caprolactone, F OPD ), molecular weight (M n ) and changes in molecular weight distribution (PD) and yield. The specific data are shown in Table 2 below:
[0065] Table 2
[0066]
[0067] a When the proportion of OPD units reaches more than 40%, the polymer is no longer soluble in THF, so there is no M for GPC test. n and PD data.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com