Selective infrared absorption material of aminodiacetic acid intercalation structure and method for preparing same
A technology of aminodiacetic acid and iminodiacetic acid, which is applied in the field of aminodiacetic acid intercalated structure selective external absorption materials and its preparation, can solve the problems of easy decomposition, accelerated destruction of material properties, poor thermal stability of IDAs, etc. Simple, low-cost, easy-to-prepare effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
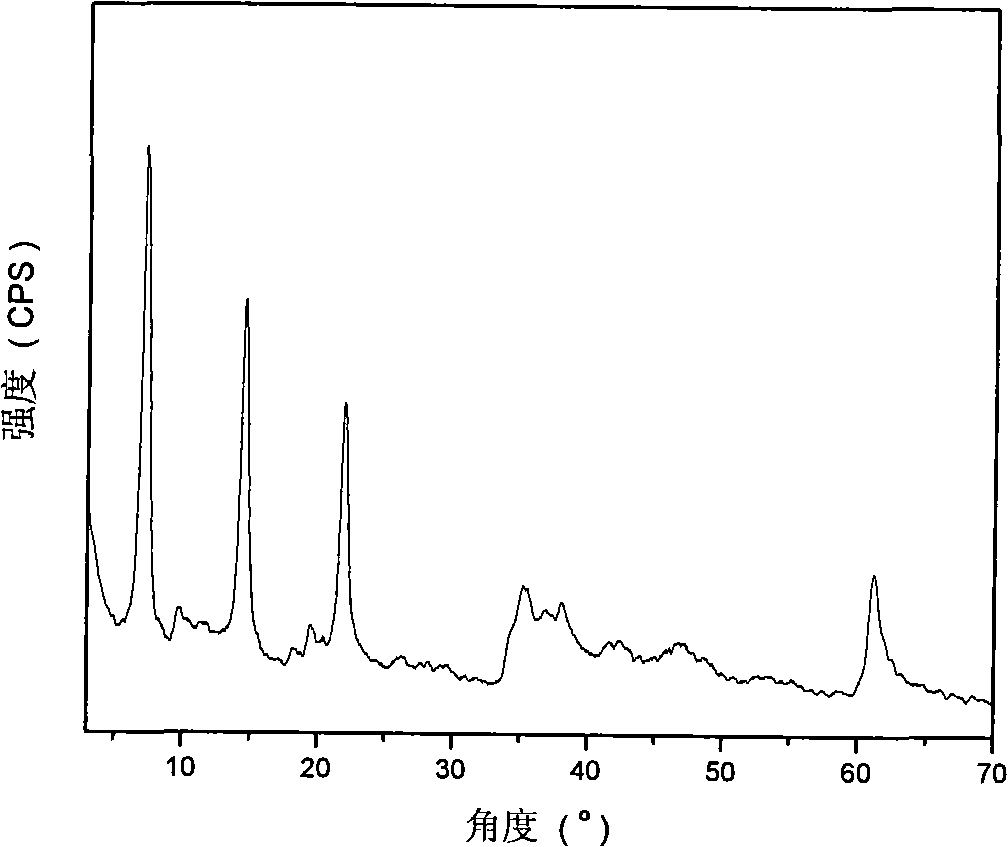
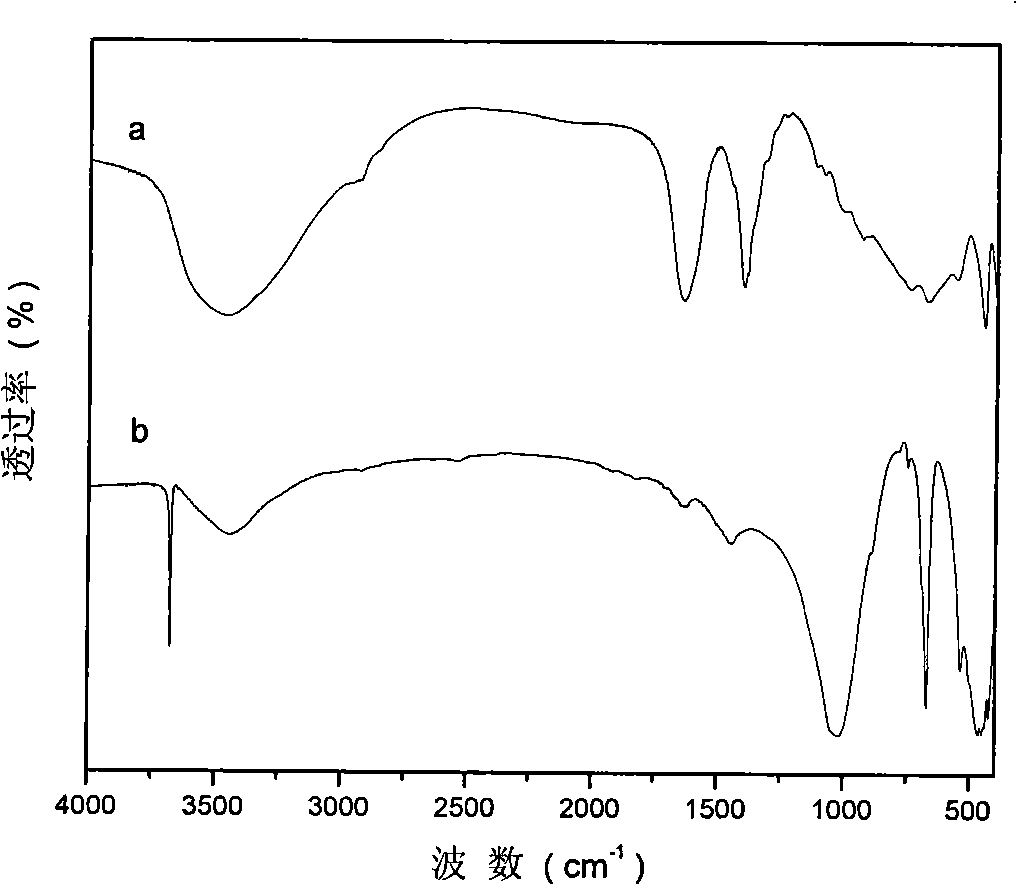
[0029] Step A: 45.0g (0.12mol) of solid Al(NO 3 ) 3 9H 2 O and 61.5g (0.24mol) of solid Mg(NO 3 ) 2 ·6H 2 O dissolves in removing CO 2 300ml of mixed salt solution A was prepared in deionized water; another 28.8g (0.72mol) of solid NaOH was dissolved in 2 300ml of alkaline solution B was prepared in deionized water. Rapidly nucleate the alkali solution and the salt solution in a fully back-mixed rotary liquid film reactor at room temperature, crystallize the obtained slurry at 100°C for 6 hours, and centrifuge to separate the obtained sample until the pH value is close to 7 to obtain hydrotalcite. body filter cake. Take a small amount and dry at 70°C for 24 hours to obtain MgAl-NO 3 -LDHs, its Mg 2+ / Al 3+ =2:1.
[0030] Take 18.9g (0.015mol) of the above filter cake to remove CO 2 Ultrasonic dispersion in deionized water to prepare a 150ml suspension.
[0031] Step B: Weigh 6.0g (0.045mol) of iminodiacetic acid (IDA) and dissolve in 2 100ml solution was prepared...
Embodiment 2
[0048] Step A: MgAl-NO 3 -The preparation of LDHs is the same as in Example 1.
[0049] Take 22.7g (0.015ml) of the above filter cake to remove CO 2 Ultrasonic dispersion in deionized water to prepare a 150ml suspension.
[0050] Step B: Weigh 8.0g (0.045ml) of N-(2-hydroxyethyl)iminodiacetic acid (HIDA) and dissolve in CO 2 150 ml solution was prepared in deionized water, and NaOH was added to adjust its pH value to 4.
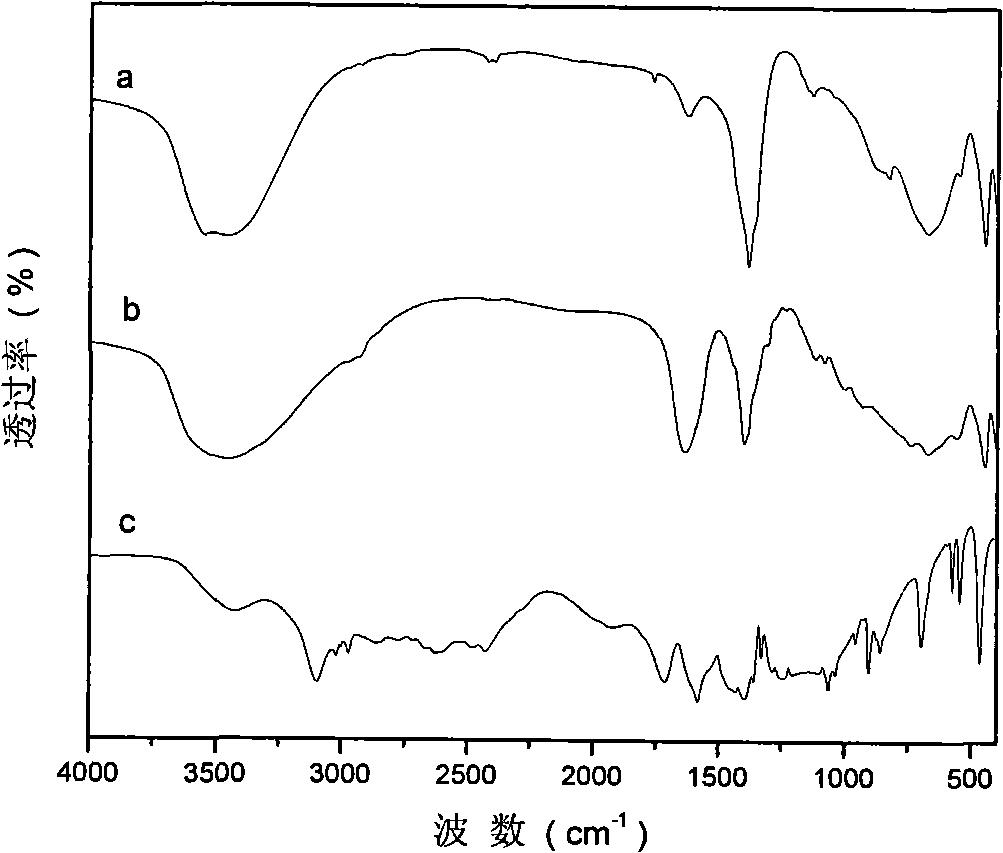
[0051] Step C: Under the protection of nitrogen, quickly add the precursor slurry prepared in step A to the solution prepared in step B, crystallize at 100°C for 6 hours, filter, and remove CO with 2 Wash with deionized water until the pH is about 7, and dry at 70°C for 24 hours to obtain MgAl-HIDA-LDHs, an infrared absorbing material with HIDA intercalation structure.
[0052] Use TG / DTA, ICP and elemental analysis methods to analyze and characterize the product, and determine its chemical formula / composition as: Mg 0.604 Al 0.396 (OH) 2 (C 6 h 9 NO...
Embodiment 3
[0054] Step A: MgAl-NO 3 -The preparation of LDHs is the same as in Example 1.
[0055] Take 25.0g (0.015ml) of the above filter cake to remove CO 2 Ultrasonic dispersion in deionized water to prepare a 150ml suspension.
[0056] Step B: Weigh 6.6g (0.045ml) of methyliminodiacetic acid (MIDA) and dissolve in 2 150 ml solution was prepared in deionized water, and NaOH was added to adjust its pH value to 4.
[0057] Step C: Under the protection of nitrogen, quickly add the precursor slurry prepared in step A to the solution prepared in step B, crystallize at 100°C for 6 hours, filter, and remove CO with 2 Wash with deionized water until the pH is about 7, and dry at 70°C for 24 hours to obtain MgAl-MIDA-LDHs, an infrared absorbing material with MIDA intercalation structure.
[0058] Use TG / DTA, ICP and elemental analysis methods to analyze and characterize the product, and determine its chemical formula / composition as: Mg 0.618 Al 0.382 (OH) 2 (C 5 h 7 NO 4 2- ) 0.18...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com