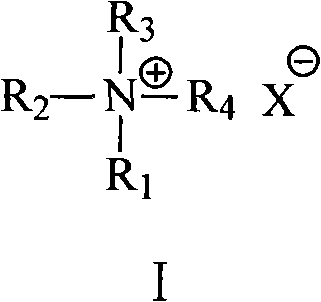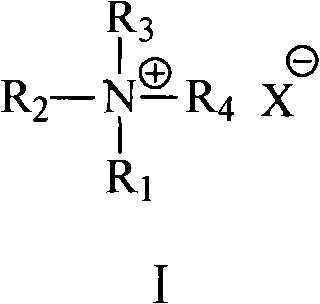Preparation of 3-methylcy-clopentadecanone
A kind of methyl cyclopentadecanone, said technology, is applied in the preparation field of 3-methyl cyclopentadecanone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
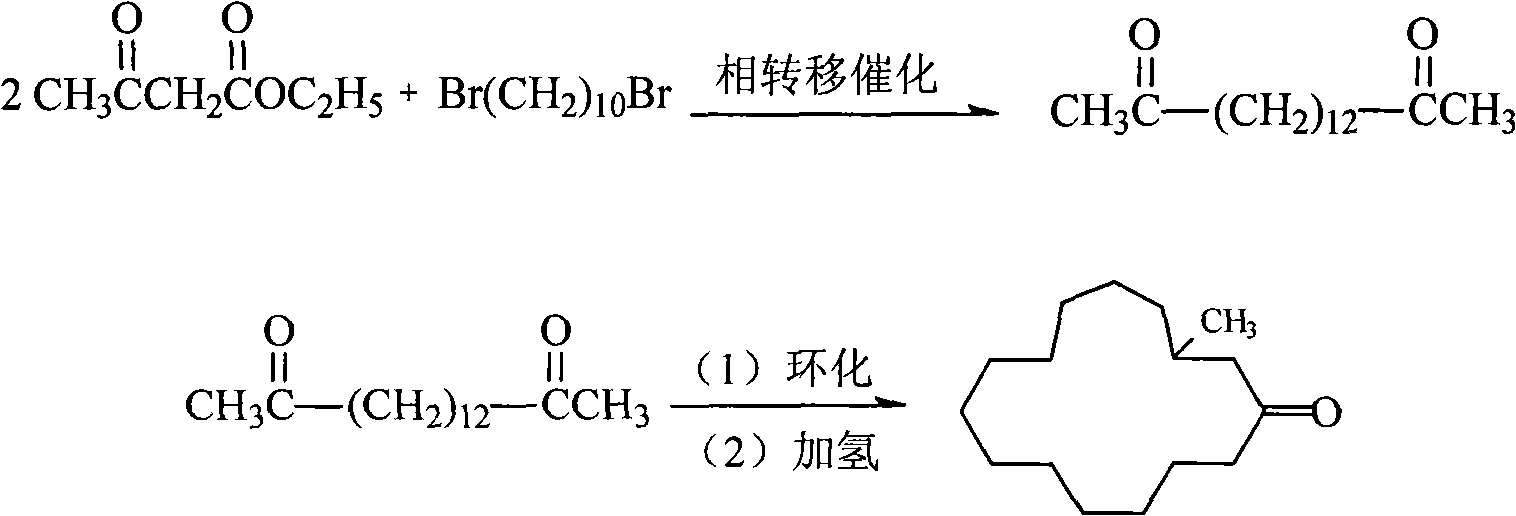
[0026] The said method of preparing 3-methylcyclopentadecone of the present invention, it comprises the steps:
[0027] (1) In the presence of an inorganic base and a compound represented by formula I, place 1,10-dibromodecane, ethyl acetoacetate and an aprotic polar solvent in a reactor at a temperature of 30°C to 150°C , the pressure is under the condition of 50kPa~1000kPa, after reacting for 0.5-30 hours, after separation (comprising the compound shown in formula I and steaming the aprotic polar solvent used), the solid is separated, and the solid is treated with alkali metal hydroxide Hydrolyze with dilute alcohol solution, filter and recrystallize to get hexadecanedione-(2,15).
[0028]Wherein: the molar ratio of 1,10-dibromodecane and ethyl acetoacetate is (1~20): 1, preferably (5~15): 1; the consumption of the compound shown in formula I is used 1,10- 2wt%~80wt% (preferably 5wt%~60wt%) of dibromodecane weight; Said inorganic base is selected from: the carbonate of alka...
Embodiment 1
[0036] (1) In a 250ml flask, add 100ml toluene, 12g (0.04mol) 1,10-dibromodecane, 4g of anhydrous potassium carbonate, 1g of dodecyltrimethylammonium chloride, 50g of ethyl acetoacetate, and use Adjust the transformer to heat it to reflux (82°C) and react for 15 hours. After the reaction was completed, it was filtered to obtain a light yellow liquid. The solvent benzene was removed under normal pressure, and ethyl acetoacetate was recovered under reduced pressure to obtain a yellow solid residue.
[0037] The yellow solid residue was placed in the flask without taking it out, and was directly used for the hydrolysis reaction. After the flask was cooled to room temperature, the prepared NaOH dilute alcohol solution (10g NaOH, 60mlH 2 (0, 25ml of absolute ethanol), heated to reflux (80° C.), and hydrolyzed for 2 hours to obtain a dark brown transparent liquid. The liquid in the flask was cooled to 50°C, poured out while hot, and cooled to obtain a solid. Filter, then dissolv...
Embodiment 2~10
[0044] Same as Example 1, reacted under different catalysts and different reaction conditions, the results are shown in Tables 1 to 3.
[0045]
[0046]
[0047]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com