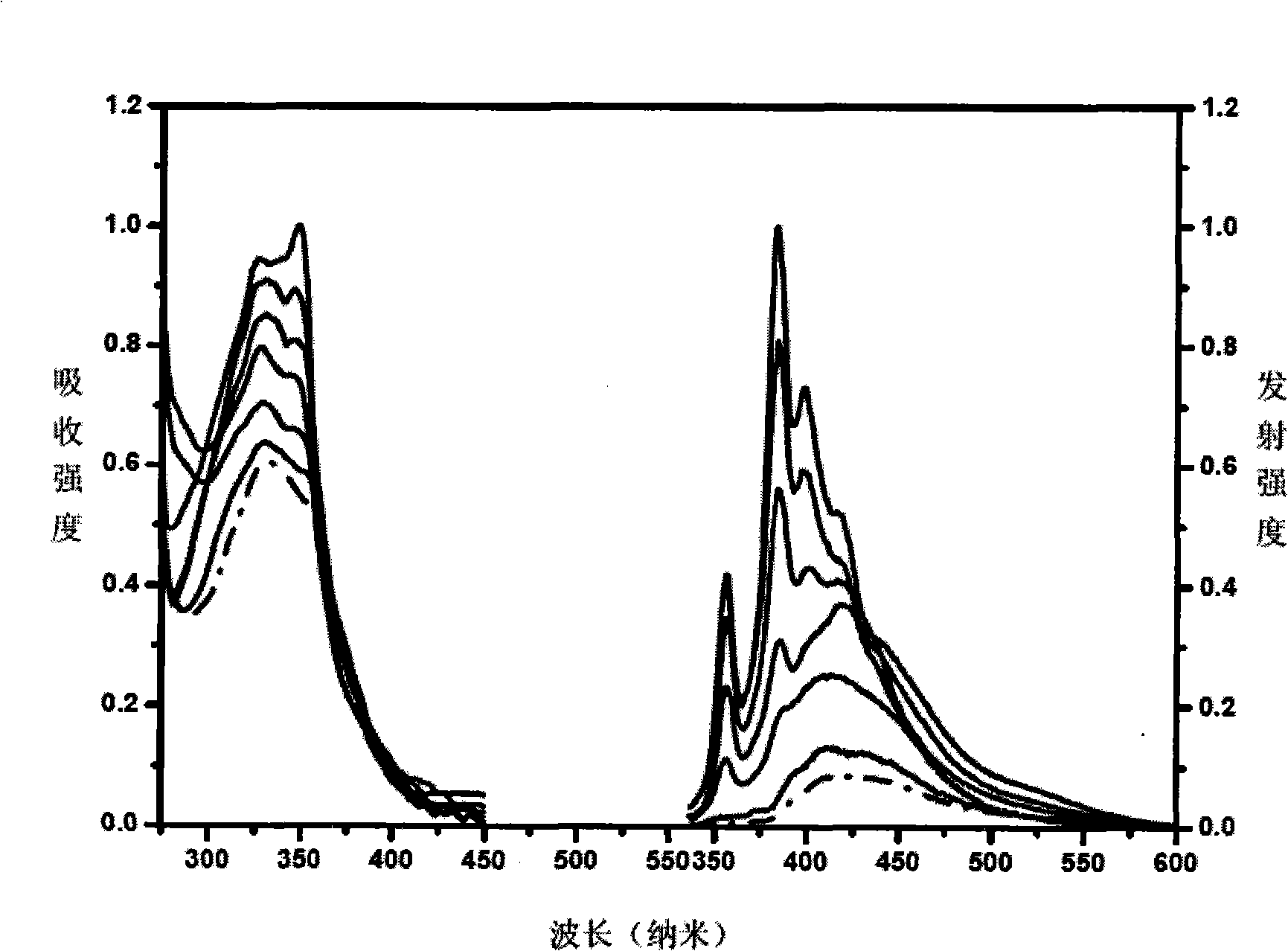
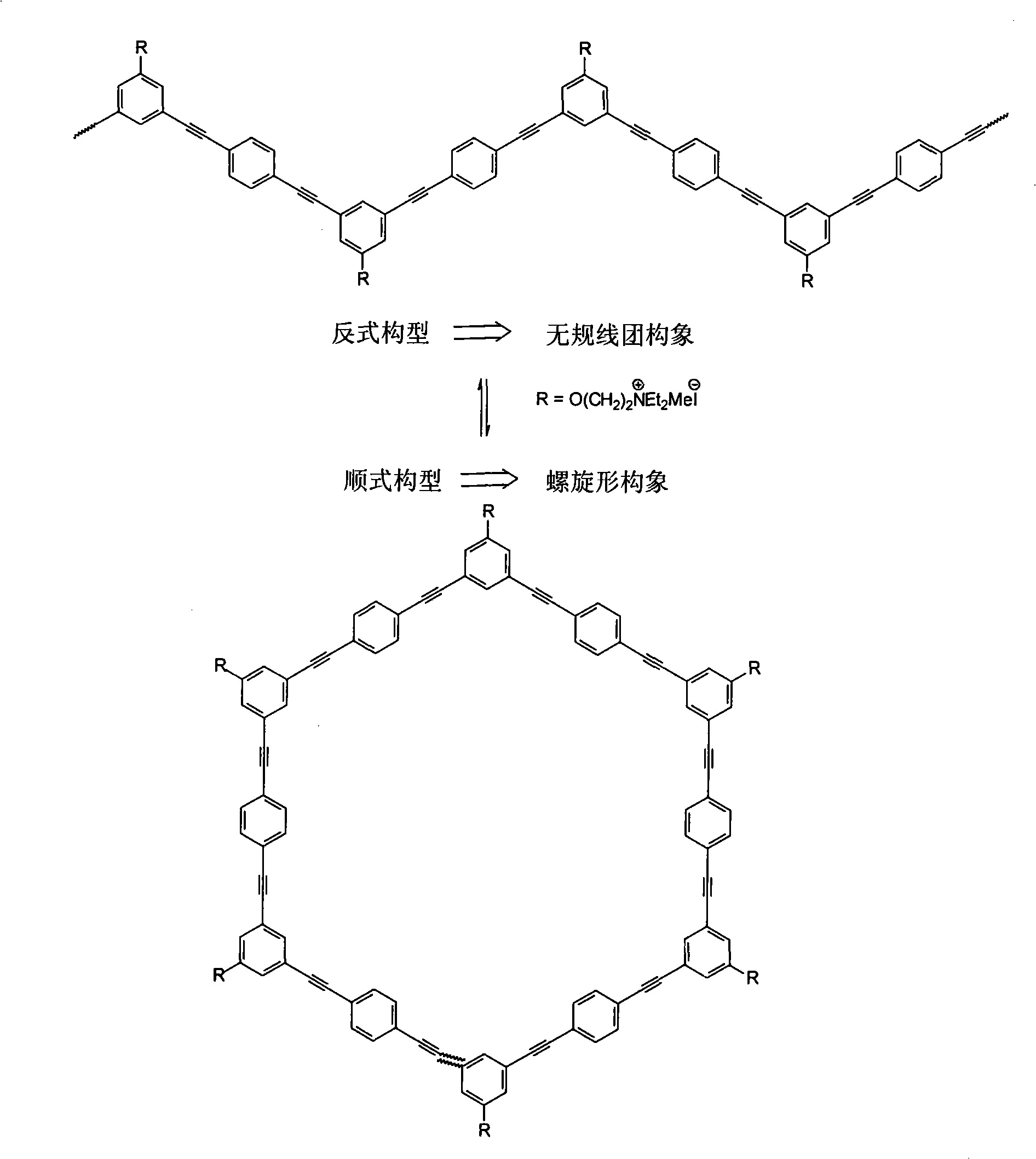
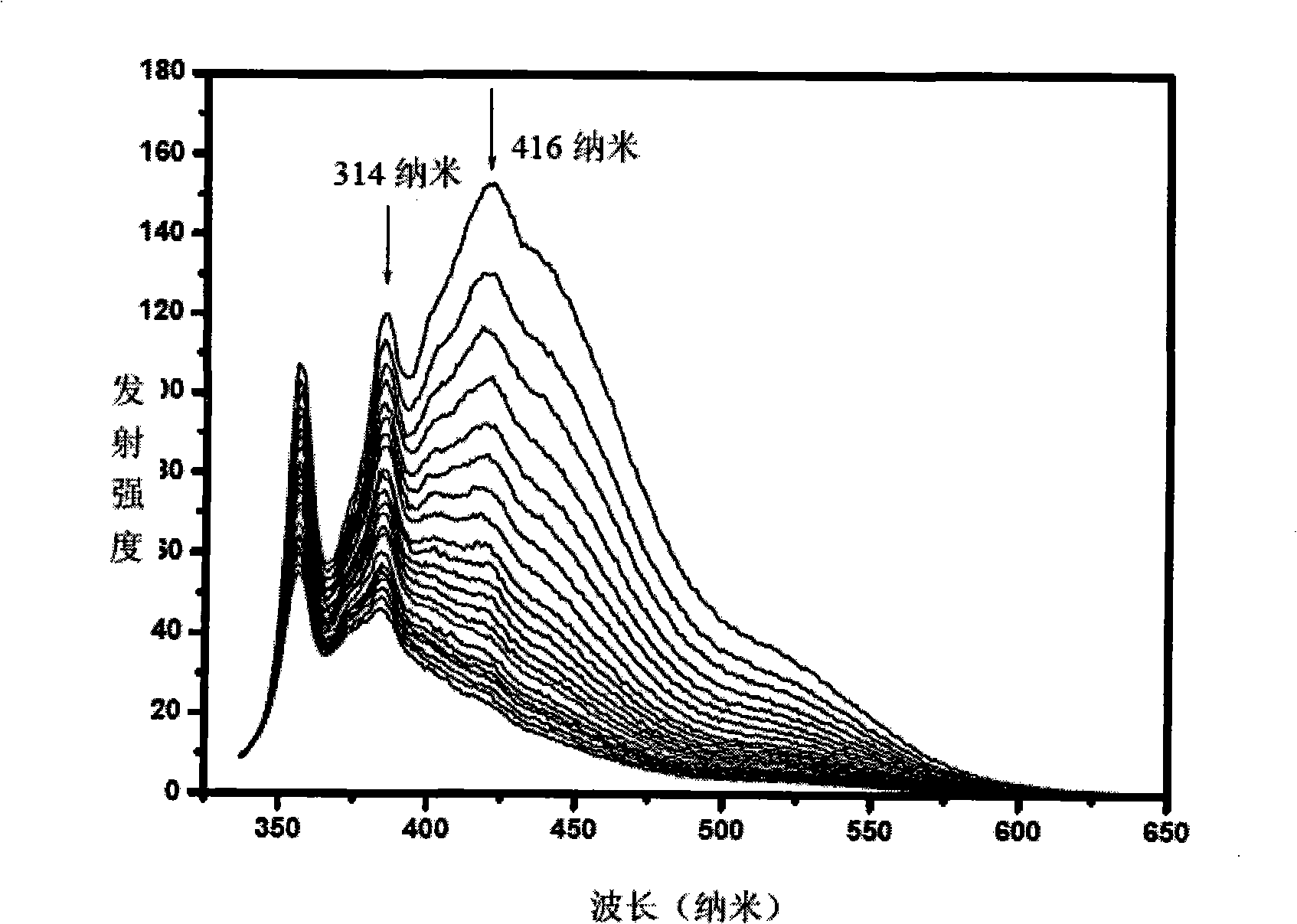
Polygonal line type water-soluble aryleneethynylene, preparation and application thereof
A water-soluble polymer and polyline technology, applied in the field of chemical/biosensing materials, can solve the problems of weakened fluorescence, insufficient interaction of biomolecules, and decreased detection sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Embodiment 1: the synthesis of cationic broken-line water-soluble poly(arylene acetylene I)
[0087] (1) Synthesis of 2,4-diiodo-6-nitroaniline (compound 2)
[0088] 39.5g (0.24mol) of iodine monochloride was dissolved in 35mL of 1,2-dichloroethane, and slowly dropped into 17g (0.12mol) of 75°C 1,2-dichloroethane (150mL) solution, A reddish-brown precipitate was produced, and the stirring was continued at this temperature for 8 hours, and then cooled to room temperature. Suction filtration, the resulting solid was washed with methanol, and the product was an orange-red solid (23 g, yield 50%). Melting point: 151°C. 1 HNMR (CDCl 3 , 400MHz): δ (ppm) 8.44 (d, 1H, J = 1.9Hz), 8.13 (d, 1H, J = 2.3Hz), 6.69 (br s, 2H). C 6 h 4 I 2 N 2 o 2 Elemental analysis theoretical value: C, 18.48; H, 1.03; N, 7.18; measured value: C, 18.64; H, 1.01; N, 7.13.
[0089] (2) Synthesis of 1,3-diiodo-5-nitrobenzene (compound 3)
[0090] At 0°C, slowly add 8.4g (0.12mol) of sodium n...
Embodiment 2
[0101] Example 2: Synthesis of cationic broken-line water-soluble poly(arylene acetylene VII)
[0102] (1) Synthesis of 2,7-diiodofluorene (compound 11)
[0103] At 80°C, fluorene (2.54g, 15mmol) was dissolved in 165mL of mixed solvent (acetic acid: water: sulfuric acid = 50:4:1), and potassium iodate (1.28g, 6mmol) and iodine (4.1g, 16.3 mmol), the mixture was stirred at this temperature for 10 hours, and precipitation occurred during the reaction. After cooling to room temperature, the precipitate was collected by suction filtration, and the solid was washed with 2M sodium carbonate solution and water until the filtrate was neutral. The crude product was recrystallized from dichloromethane to obtain a pale yellow solid (4.76 g, yield 76%). Melting point: 216-217°C. 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 7.87 (s, 2H), 7.70 (d, 2H, J=8.0Hz), 7.49 (d, 2H, J=8.0Hz), 3.83 (s, 2H). C 13 h 8 I 2 Elemental analysis theoretical value: C, 37.35; H, 1.93; measured value: C, 37.36; ...
Embodiment 3
[0114] Embodiment 3: Synthesis of anionic broken-line water-soluble poly(arylene acetylene II)
[0115] (1) Synthesis of m-diiodobenzene (monomer F) whose side chain end group is a sulfonic acid group
[0116] Dissolve 3.5g (0.022mol) of sodium hydroxide in 50mL of ethanol, add dropwise to 6.92g (0.02mol) of 3,5-diiodophenol (compound 5) in 50mL of ethanol solution, and stir for 1 hour. Dissolve 2.68g (0.022mol) of 1,3-propane sultone in 50mL of ethanol, add the above solution, and stir for 12 hours at a reaction temperature of 50-60°C. The crude product was obtained by suction filtration, and the solid was recrystallized from absolute ethanol and water to obtain monomer F with a yield of 56%. 1 HNMR (DMSO-d 6 , 400MHz): δ (ppm) 7.58 (s, 1H), 7.14 (s, 2H), 3.94 (t, 2H), 3.41 (t, 2H), 2.27 (m, 2H).
[0117] (2) Synthesis of broken-line water-soluble poly(arylene acetylene II)
[0118] Under the protection of nitrogen, add the mixed solvent diisopropylamine, water and N,N-di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com