Method for preparing cupric oxide nano-hollow ball with forerunner reaction
A precursor, copper oxide technology, applied in the field of nanomaterials, can solve problems such as blank hollow spheres, and achieve the effects of low price, avoiding the introduction of impurities, and good retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
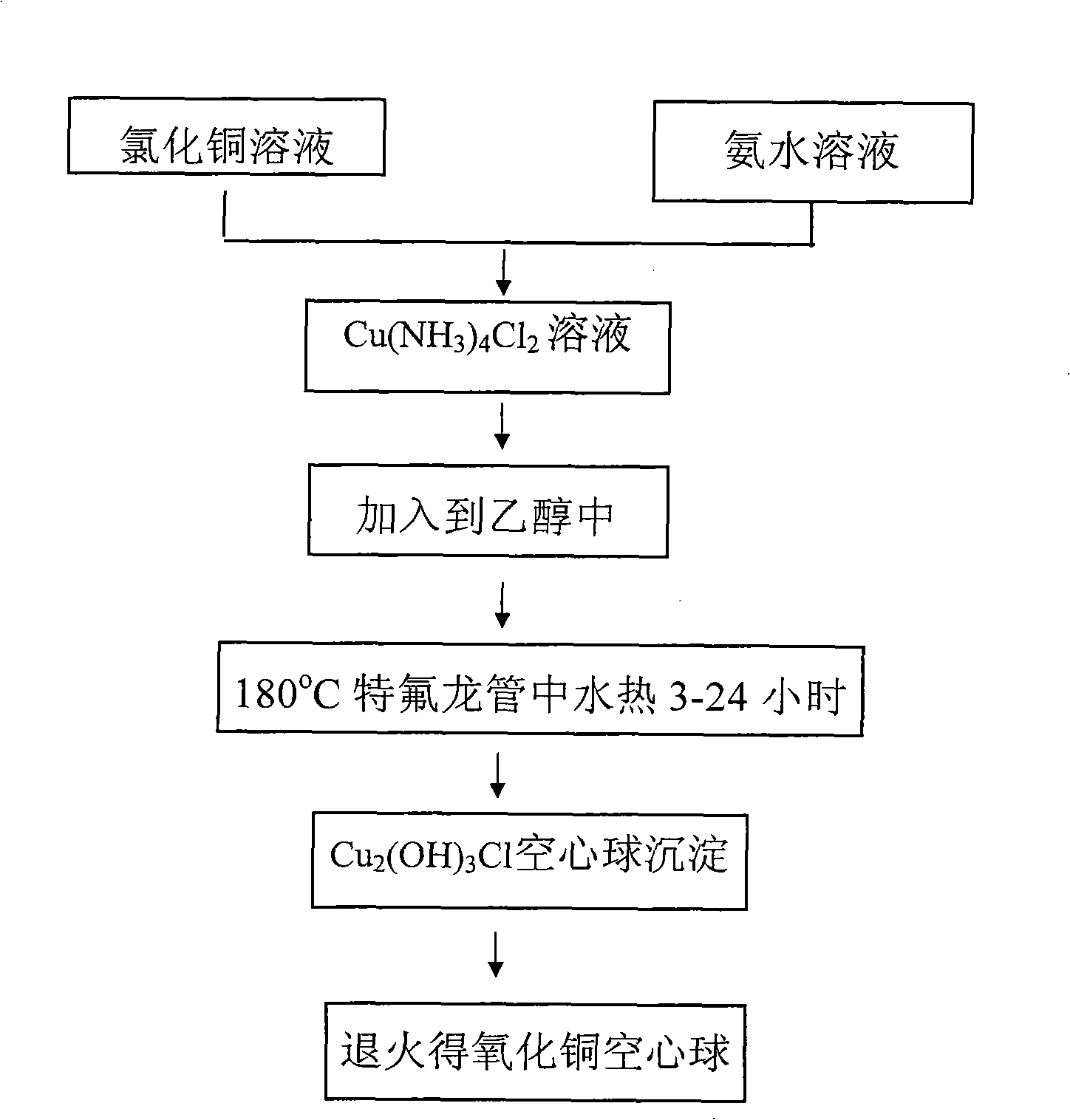
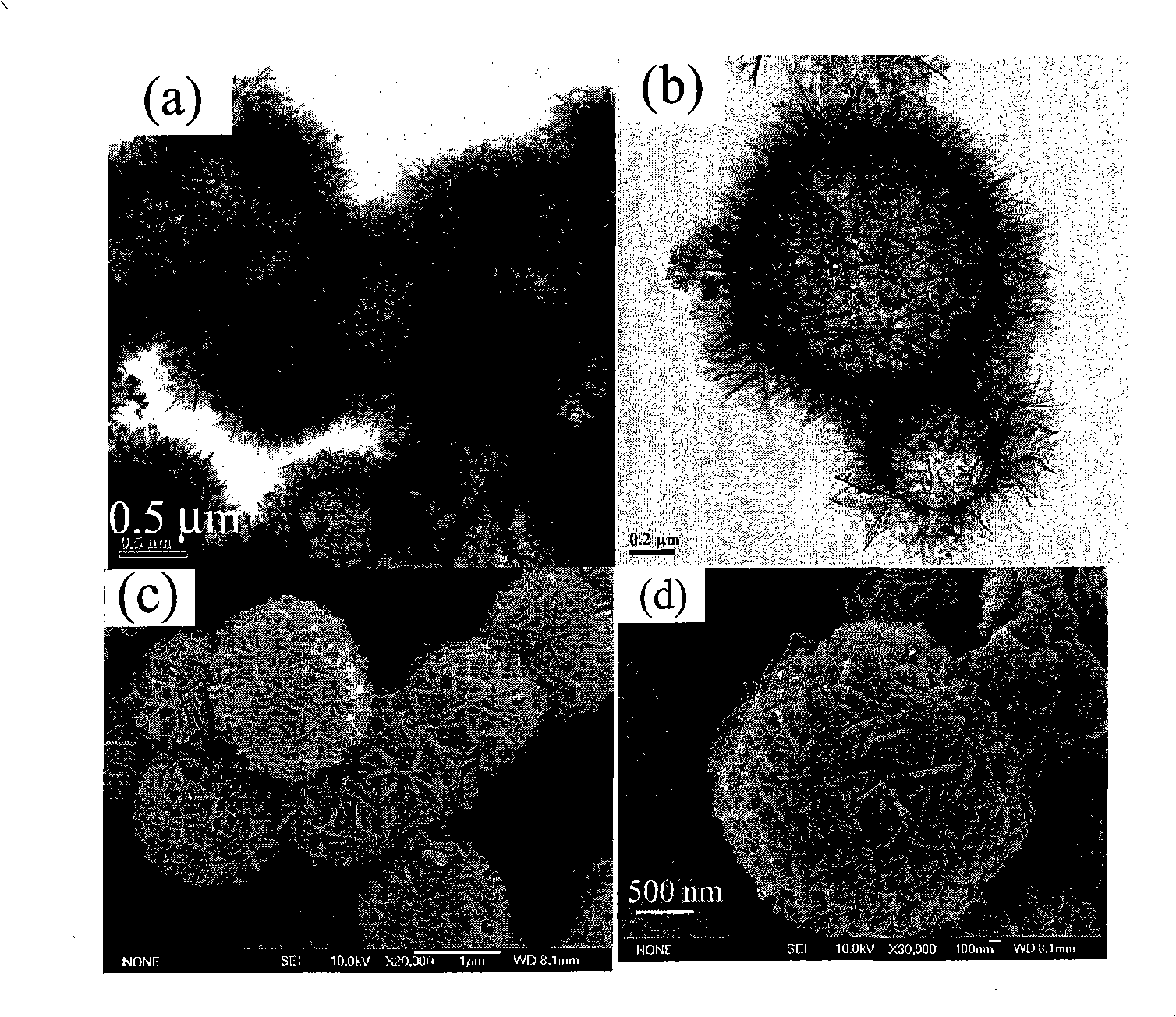
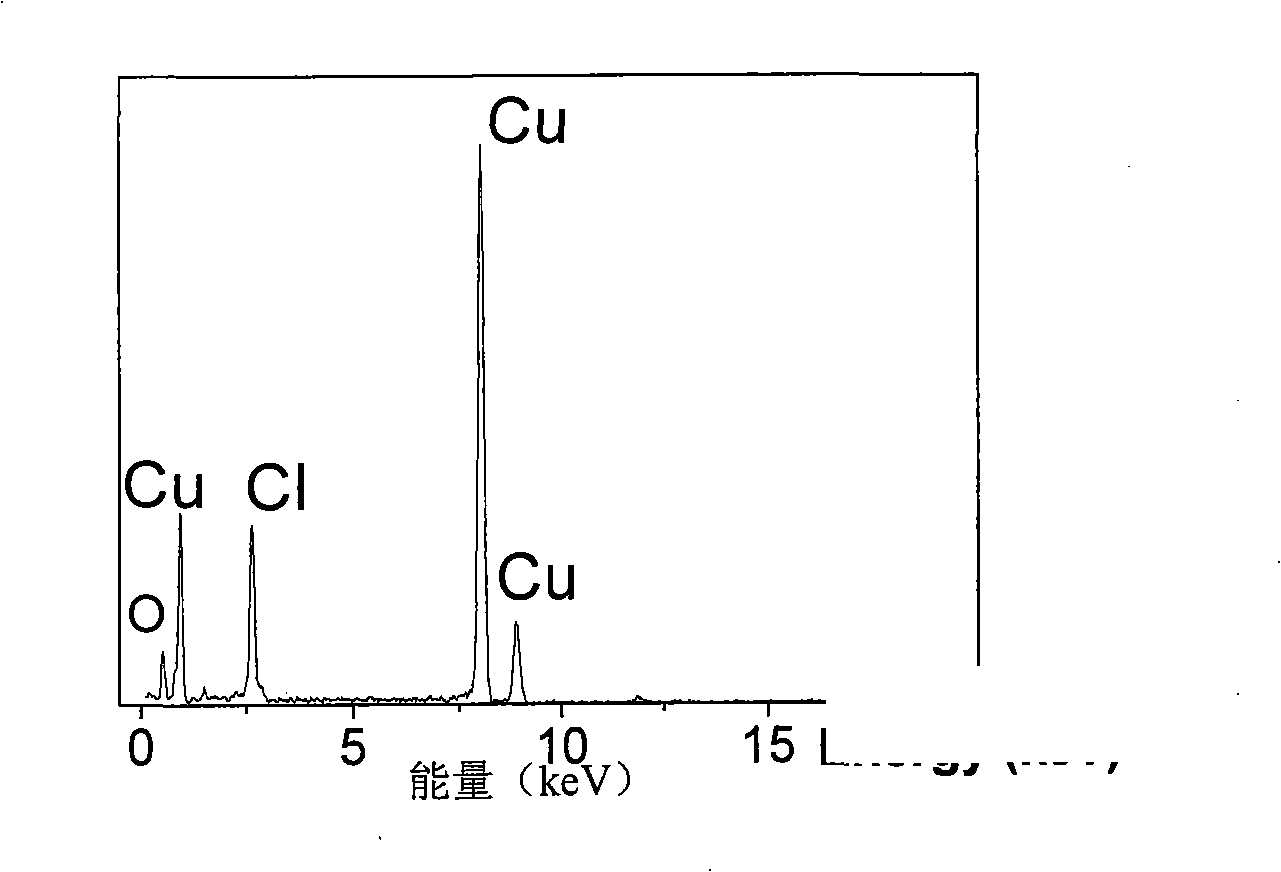
[0035] First prepare solution A: 3.41g CuCl 2 2H 2 O was dissolved in 100 mL of deionized water and dissolved under stirring at 300-500 rpm. At the same time, solution B: 5M concentrated ammonia solution was prepared. CuCl in A solution 2 After stable dissolution and complete transparency, add solution B dropwise under magnetic stirring at 300-600 rpm until PH=10-14. Due to the addition of ammonia water, solution A immediately produces a blue precipitate. With the gradual addition of ammonia water under magnetic stirring, the blue precipitate dissolves rapidly, forming a stable blue Cu(NH 3 ) 4 2+ of ammonia solution. Cu(NH 3 ) 4 2+8 mL of the solution was added to 75 mL of absolute ethanol, and transferred to a 100 mL Teflon tube with a volume ratio of 82%. React at 180°C for 12h to get green Cu 2 (OH) 3 Precipitation of Cl nanohollow spheres. Repeat washing with deionized water and absolute ethanol 5 times each, and dry under vacuum at 50°C for 4 hours. The pel...
Embodiment 2
[0037] First prepare solution A: 3.41g CuCl 2 2H 2 O was dissolved in 100 mL of deionized water and dissolved under stirring at 300-500 rpm. At the same time, solution B: 5M concentrated ammonia solution was prepared. CuCl in A solution 2 After stable dissolution and complete transparency, add solution B dropwise under magnetic stirring at 300-600 rpm until PH=9.5. Due to the addition of ammonia water, solution A immediately produces a blue precipitate. With the gradual addition of ammonia water under magnetic stirring, the blue precipitate dissolves rapidly, forming a stable blue Cu(NH 3 ) 4 2+ of ammonia solution. Cu(NH 3 ) 4 2+ Add 8mL of the solution to 75mL of absolute ethanol, transfer to a 100mL Teflon tube, and react at 180°C for 12h to obtain green Cu 2 (OH) 3 Cl precipitates. Repeat washing with deionized water and absolute ethanol 5 times each, and dry under vacuum at 50°C for 4 hours. The pellet was then annealed at 400°C for 2 hours. Figure 5 It is ...
Embodiment 3
[0039] First prepare solution A: 3.41g CuCl 2 2H 2 O was dissolved in 100 mL of deionized water and dissolved under stirring at 300-500 rpm. At the same time, solution B: 5M concentrated ammonia solution was prepared. CuCl in A solution 2 After stable dissolution and complete transparency, add solution B dropwise under magnetic stirring at 300-600 rpm until PH=12. Due to the addition of ammonia water, solution A immediately produces a blue precipitate. With the gradual addition of ammonia water under magnetic stirring, the blue precipitate dissolves rapidly, forming a stable blue Cu(NH 3 ) 4 2+ of ammonia solution. Cu(NH 3 ) 4 2+ Add 3mL of the solution to 77mL of absolute ethanol, transfer to a 100mL Teflon tube, and react at 180°C for 12h to obtain green Cu 2 (OH) 3 Cl precipitates. Repeat washing with deionized water and absolute ethanol 5 times each, and dry under vacuum at 50°C for 4 hours. The pellet was then annealed at 400°C for 2 hours. Figure 6 It is t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com