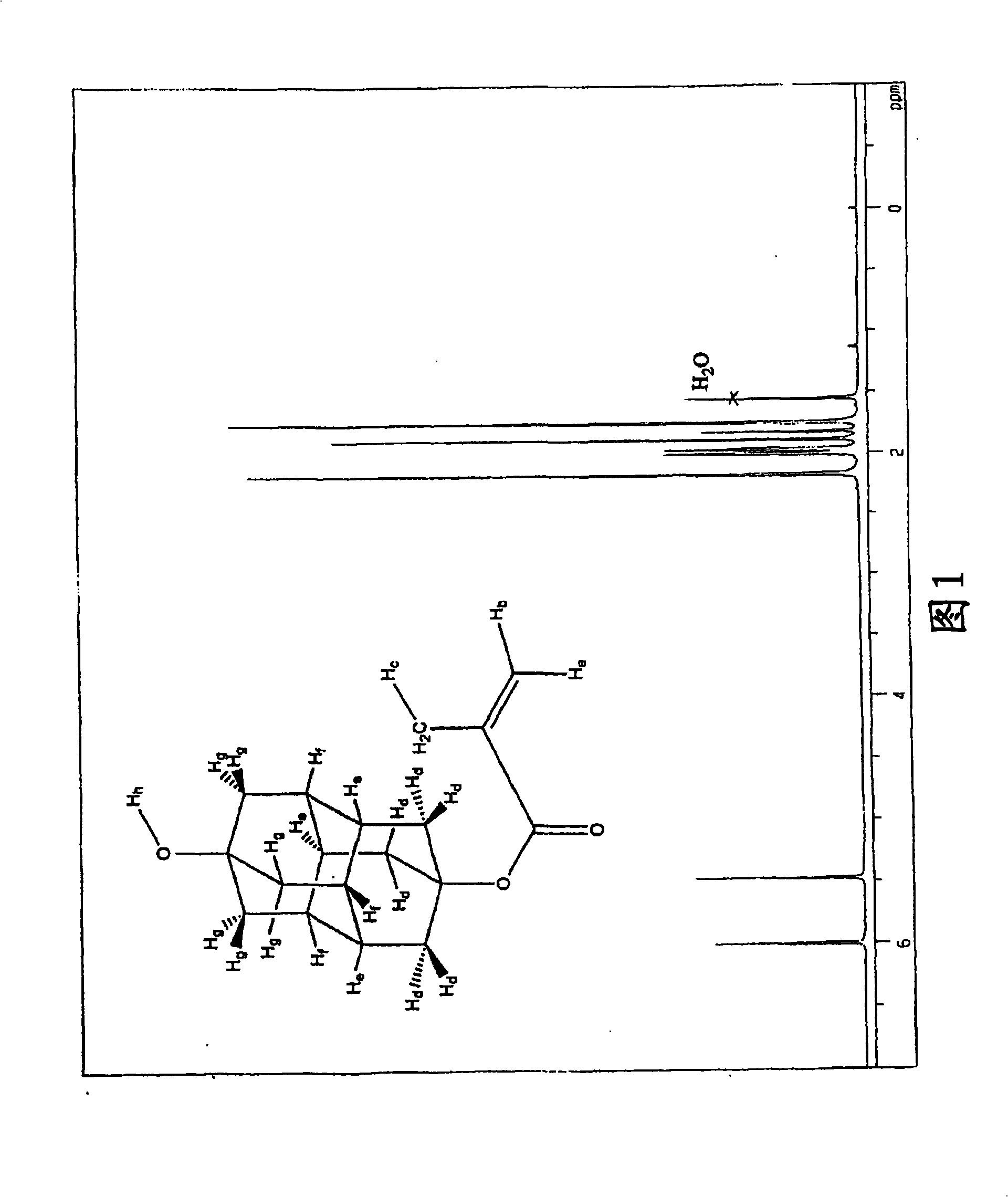
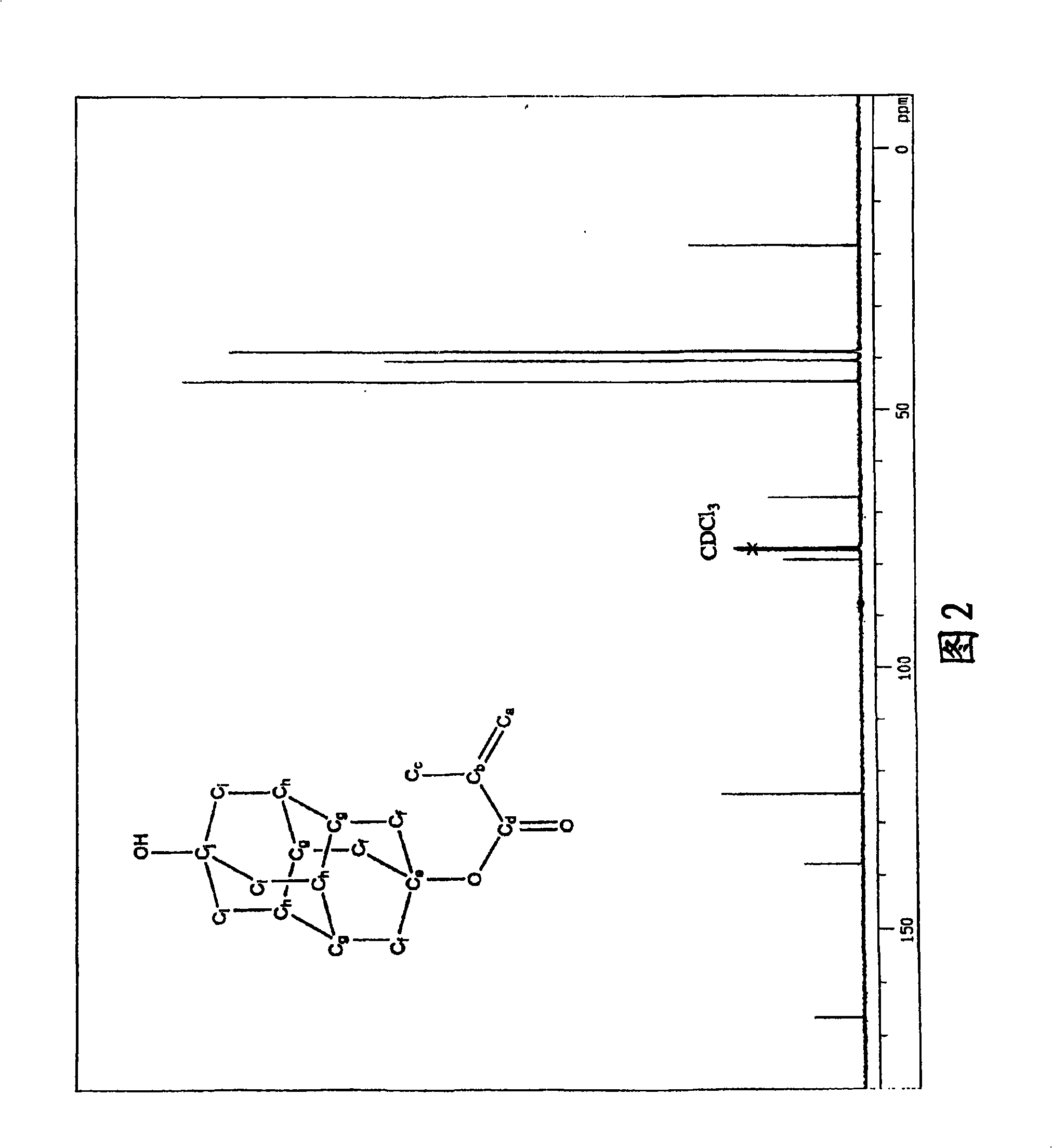
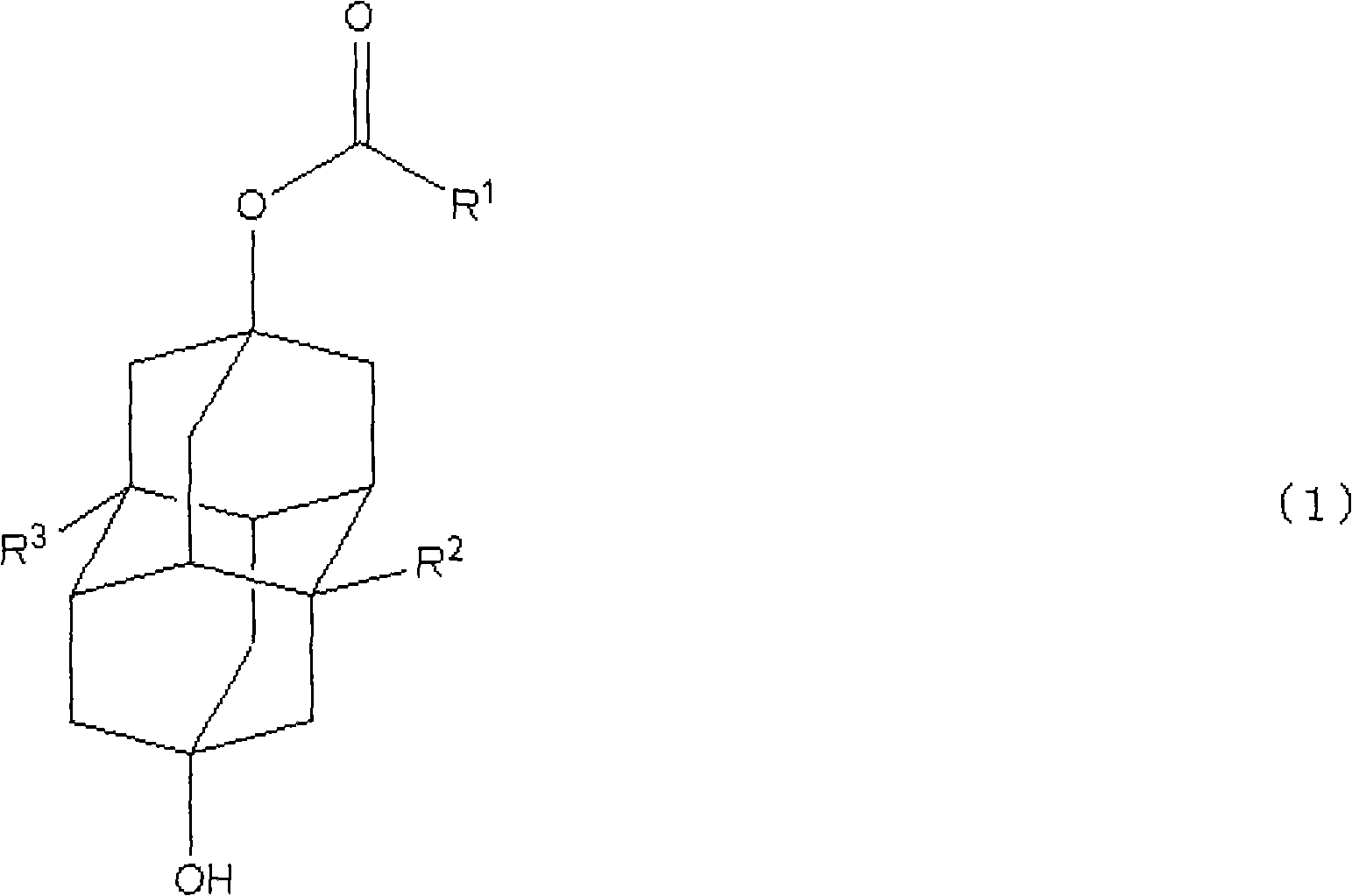
Method for producing polymerizable hydroxydiamantyl ester compound
A technology of hydroxydiadamantyl ester and dihalogenated diamantane, which is applied in the field of preparation of polymeric hydroxydiadamantyl ester compounds, and can solve problems such as difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
In the production method of the present invention, first, a diadamantane compound represented by the following formula (2) is subjected to a dihalogenation reaction to obtain a 4,9-dihalogenated diadamantane compound.
[0031] [chemical 4]
[0032] In the above formula (2), R 2 and R 3 Each independently represents a hydrogen atom or an alkyl group having 1 to 5 carbon atoms.
[0033] Specific examples of preferred groups among the alkyl groups having 1 to 5 carbon atoms include methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, tert-butyl, n-pentyl Base, sec-pentyl, isopentyl, etc.
[0034] In this step (i), as R 2 and R 3 , as long as it matches with the target polymerizable hydroxydiamantyl ester compound and selects appropriately. Specific examples of diadamantane compounds that can be used include diadamantane, 1-methyldiamantane, 1-ethyldiamantane, 1,6-dimethyldiamantane, 1,6 -Diethyldiadamantane and the like. Among these, diamantane is particularly prefera...
Embodiment 1
[0133] Add chlorosulfonic acid 222.7g (1.912mol, be 3 mole times of raw material diadamantane) (total be 5 mole times of diadamantane) to wherein, stir again at 30 ℃ for 15 hours (reaction time of 18 hours in total) ). After 15 hours, GC analysis revealed that the product contained 1% of the raw materials diadamantane, 5% of monochlorodiamantane, 80% of dichlorodiamantane, and 14% of trichlorodiamantane. After cooling the reaction liquid to about 10°C, 262 g of water was dropped while keeping the reaction liquid at a temperature not higher than 30°C. 840 g of dichloromethane was added, stirred, left to stand, and liquid-separated, and the lower sulfuric acid aqueous solution layer was removed. Then, the organic layer was washed 2 times with 240 g of 10% aqueous sodium hydroxide solution, 1 time with 300 g of 7% aqueous sodium sulfate solution, and 2 times with 120 g of 7% aqueous sodium sulfate solution. At this time, the pH value of the organic layer had changed into neutra...
Embodiment 2~3
[0142] Table 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com