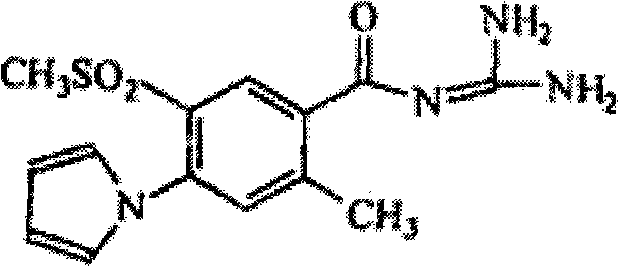
Levosimendan emulsion for intravenous injection and preparation thereof
A technology of oil for injection and water for injection, which is applied in the field of medicine, can solve the problems of levosimendan difficulty, obstruction of blood circulation, patient irritation and side effects, and achieve the effects of reducing irritation, improving compliance and increasing safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
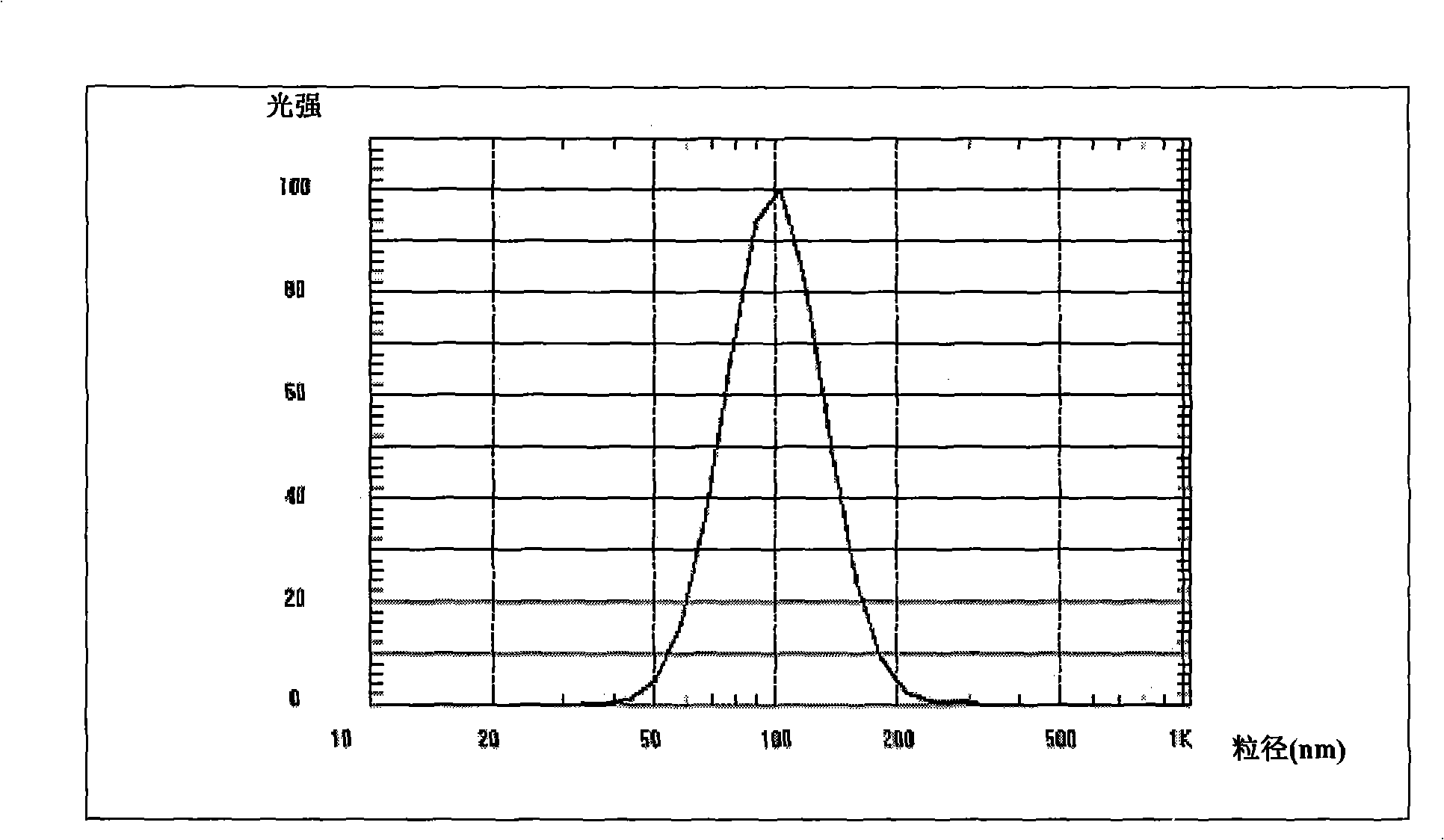
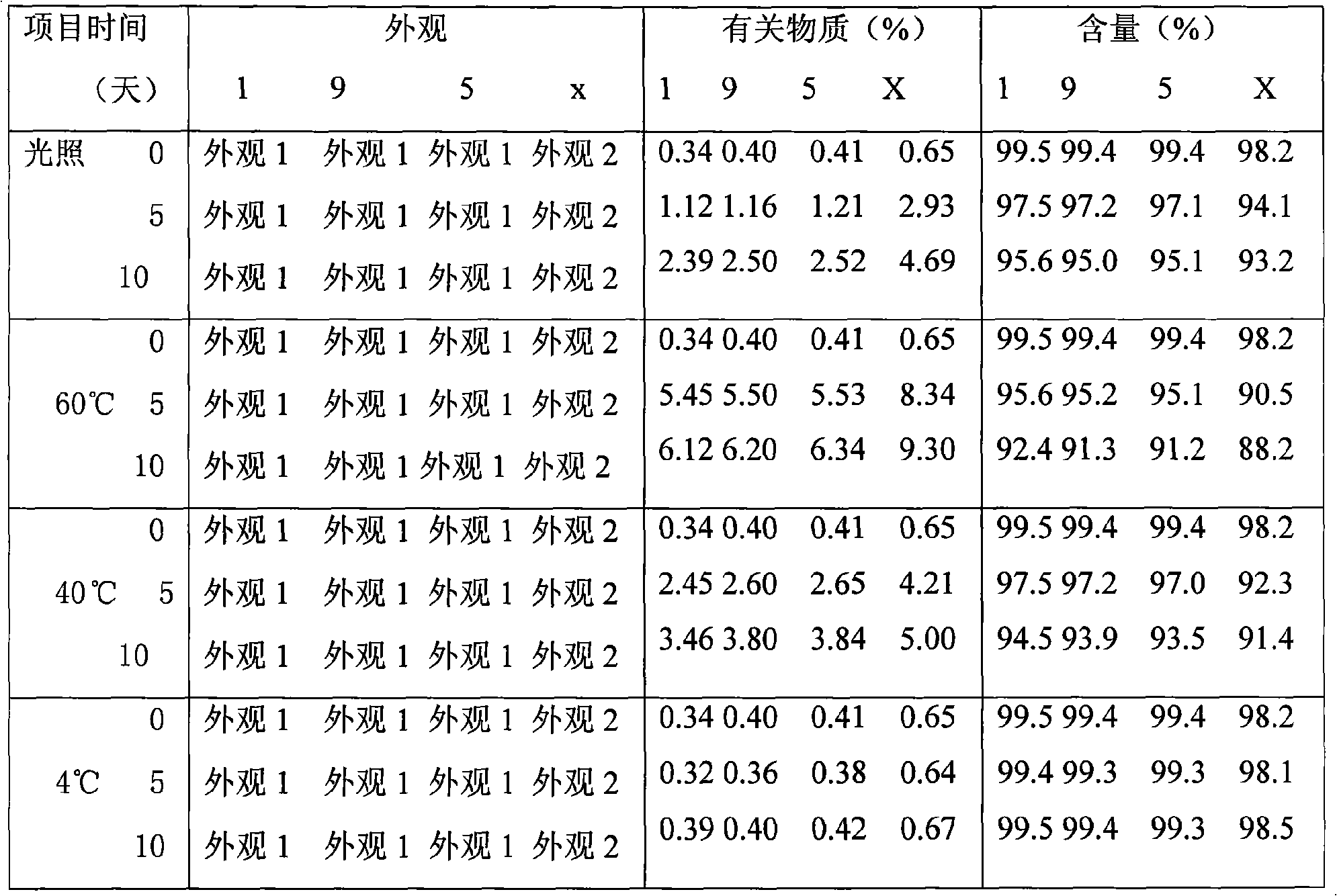
[0047] Under the protection of nitrogen, weigh 0.25g of levosimendan, 5g of lecithin, 15g of soybean oil, and 0.5g of oleic acid, heat and mix evenly to obtain an oil phase; take 2g of glycerin, 10g of sucrose and add 80ml of water for injection to dissolve, add the water phase to In the oil phase, stir at high speed, then add water for injection to 100ml, adjust the pH to 3-4.5, and continue stirring to prepare colostrum. Repeated homogenization by a high-pressure homogenizer, and checking the size of the obtained emulsion particles, the average particle size is 104.8nm. The emulsion is sterile filtered, filled, sealed, and ready after passing the inspection.
Embodiment 2
[0049] Under nitrogen protection, weigh 0.5g of levosimendan, 20g of medium-chain triglyceride, 5g of polyoxyethylene castor oil, and 0.5g of oleic acid, and mix them uniformly to obtain an oil phase; weigh PEG4005g, glucose 8g, EDTA-2Na0. 0.05 g was dissolved in 80 ml of water for injection, the water phase was added to the oil phase, stirred at high speed, then water for injection was added to 100 ml, the pH was adjusted to 3-4.5, and the colostrum was prepared by continuing to stir. Repeated homogenization by a high-pressure homogenizer, and checking the size of the obtained emulsion particles, the average particle size is 191.2nm. The emulsion is sterile filtered, filled, sealed, and ready after passing the inspection.
Embodiment 3
[0051] Under the protection of nitrogen, weigh 25g of cottonseed oil for injection and heat it to 60°C. Under high-speed stirring, add 5g of soybean lecithin, 0.5g of Tween-800.5g, and 0.7g of levosimendan, and stir evenly as the oil phase; Add 80ml of water for injection to 15g to dissolve, add the water phase to the oil phase, stir at high speed, then add water for injection to 100ml, adjust the pH to 3-4.5, and continue to stir to prepare colostrum. Repeated homogenization by a high-pressure homogenizer, and checking the size of the obtained emulsion particles, the average particle size is 204.3nm. The emulsion is sterile filtered, filled, sealed, and ready after passing the inspection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com