Tissue engineering trachea implant and construction method and use
A technology of tissue engineering and grafting, which is applied in the field of medicine and biomedical engineering, and can solve the problems of mixing mouse cells, failing to show the superiority of tissue engineering technology, and insufficient cartilage support strength, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
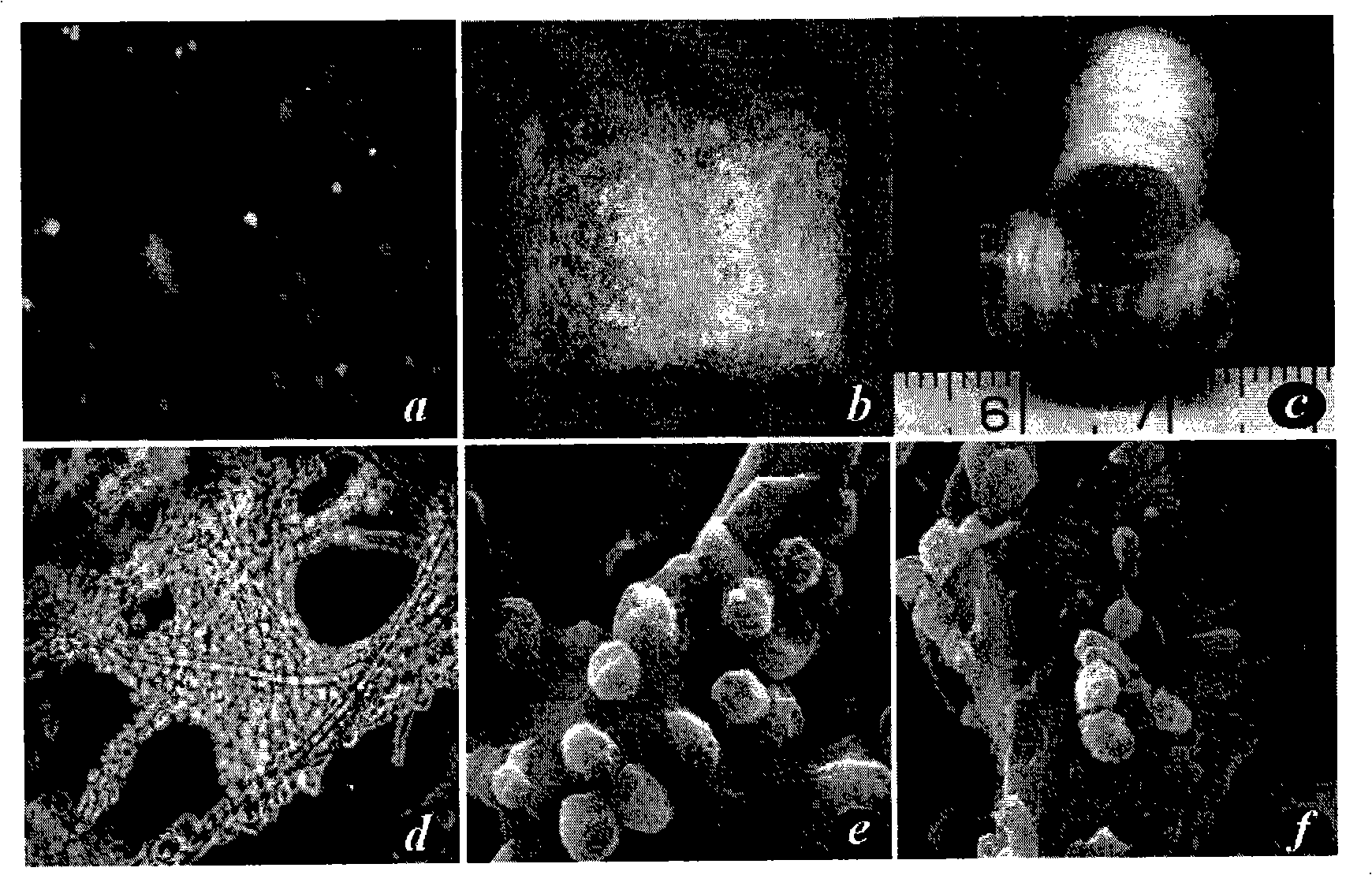
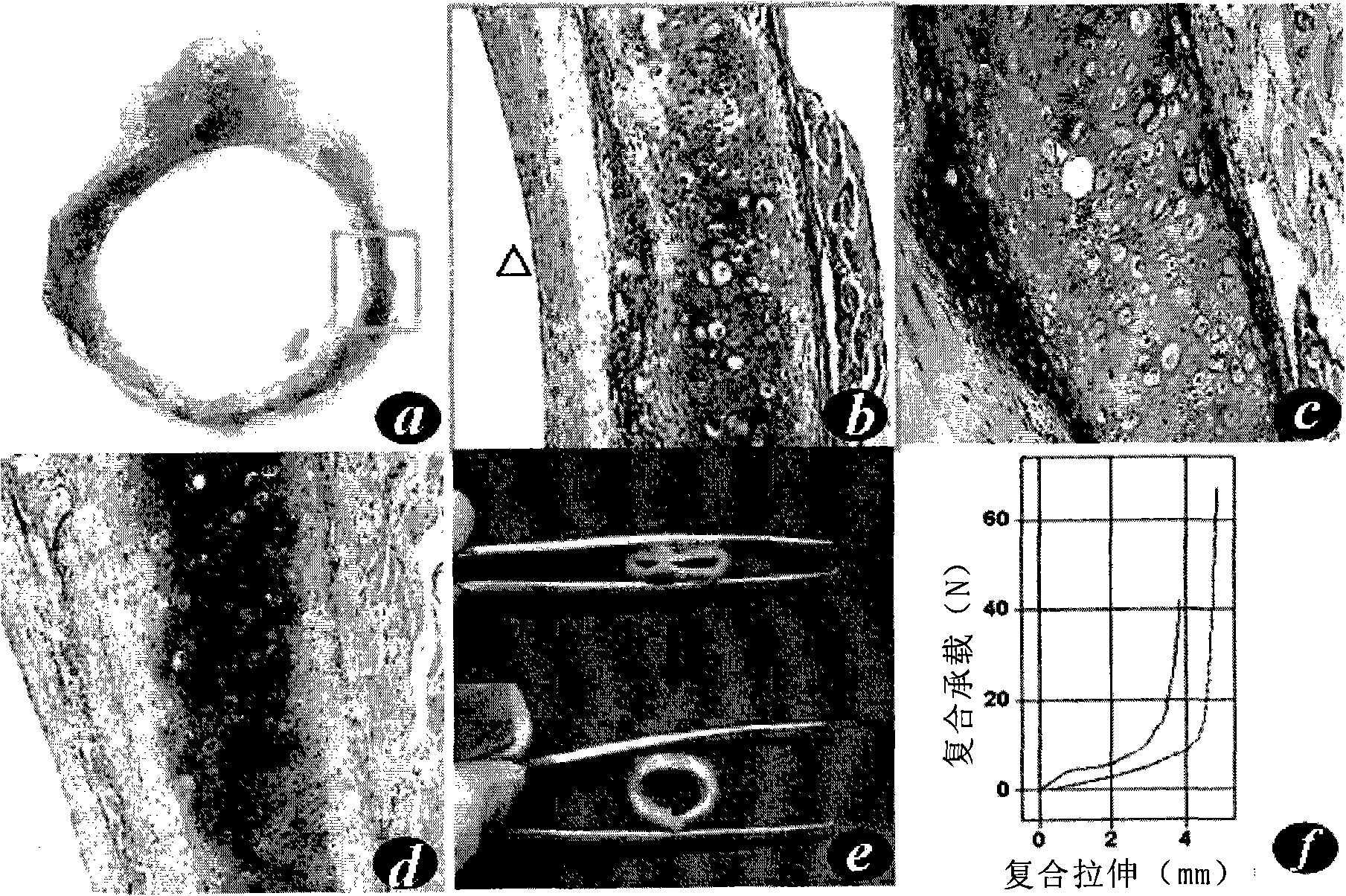
[0107] Cell Isolation and Culture
[0108] Sterilize appropriate amount of cartilage, cut into 1mm pieces 3 Cartilage particles of large or small sizes were first digested with 0.05% trypsin solution at 37°C for 30 minutes with shaking, then washed with PBS and added with 0.25% type II collagenase solution (Sigma Company), and digested with constant temperature shaking at 37°C for 7-8 hours. The digestive solution was filtered through a 100 μm filter, and after centrifugation, the cell pellet was resuspended in chondrocyte culture medium, counted, inoculated into a 10mm culture dish (DownCorning Company) at a cell density of 1.0×104 / cm2, and chondrocyte culture medium was added; the cells were incubated at 37°C , 5% CO2, cultured under saturated humidity conditions, the first medium was changed after 3 days, and the medium was changed every other day thereafter. The formula of chondrocyte culture medium is: DMEM (Dulbecco'smodified Eagle's medium) culture medium (Gibco Compan...
Embodiment 2
[0111] Scaffold material preparation
[0112] Take 60 mg of non-woven polyglycolic acid fiber (polyglycolic acid, PGA, self-developed), and spirally wind it on a silicone tube with a length of 1.5 cm and a diameter of 6 mm to form a cylindrical scaffold material ( figure 1 ), both ends were sutured and fixed with absorbable sutures, soaked in 75% alcohol for 60 minutes for disinfection, rinsed with PBS twice and then set aside.
Embodiment 3
[0114] Cell-material complex formation and in vitro culture
[0115] Chondrocytes adjusted cell concentration to 60×10 6 / ml, take 1ml of cell suspension and inoculate it evenly on the spiral material, place it at 37°C, 5% CO2, and saturated humidity for 4 hours, then transfer it into a 50ml polypropylene centrifuge tube (BD Biosciences), add 30ml of chondrocyte culture medium, and change it every other day The culture was continued for 2 weeks under the above conditions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com