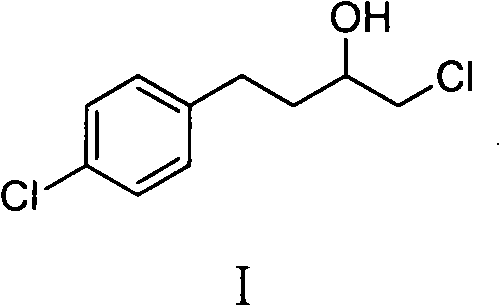
Preparation of butoconazole nitrate intermediate
A compound and reaction technology, which is applied in the field of preparation of antifungal drug butoconazole nitrate, can solve the problems of being unsuitable for large-scale industrial production, difficult to control, poor safety, etc., and achieves the effects of being beneficial to industrial production, product separation, and product ease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 15.4g of magnesium powder, 1g of methyl iodide and 250ml of tetrahydrofuran into a 2000ml three-necked flask and stir. Heat to reflux. Under reflux conditions, a mixture of 112 g of p-chlorophenethyl chloride and 500 ml of tetrahydrofuran was added dropwise to initiate the reaction. Heating was stopped and the residue was added dropwise. After the dropwise addition, the reaction was heated to reflux for 1 hour and cooled to room temperature.
[0020] Add 50.2g of chloroacetaldehyde and 250ml of tetrahydrofuran into a 3000ml three-necked flask and stir. The prepared Grignard reagent was added dropwise, the reaction was exothermic, and the solution was kept under reflux and added dropwise. After the dropwise addition was completed, it was heated to reflux for 1 hour. Add ice water and 1M hydrochloric acid, and separate the layers. The organic phase was dried with anhydrous magnesium sulfate, concentrated under reduced pressure, and the fraction at 134-138° C. (2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com