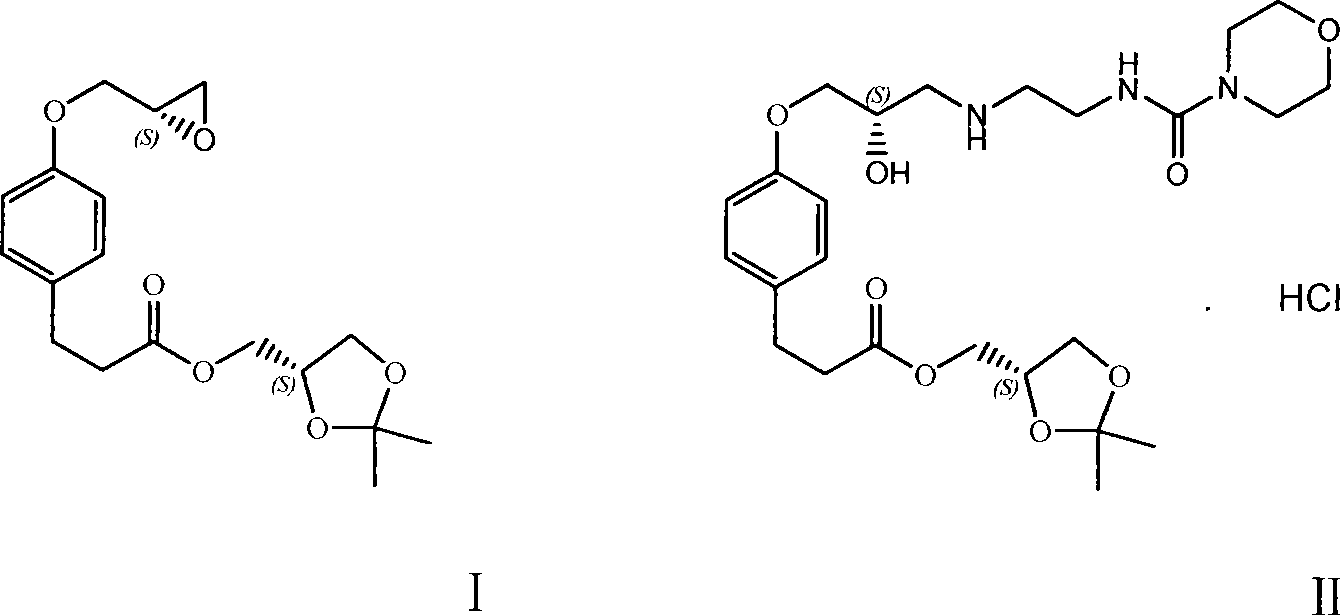
Method for preparing landiolol intermediate
A technology for compounds and heterocycles, applied in the field of preparing methyl-3-[4-, can solve the problems of high cost of target products, difficult industrial production, expensive docking raw materials, etc., and achieves low cost, easy industrial production, and high purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
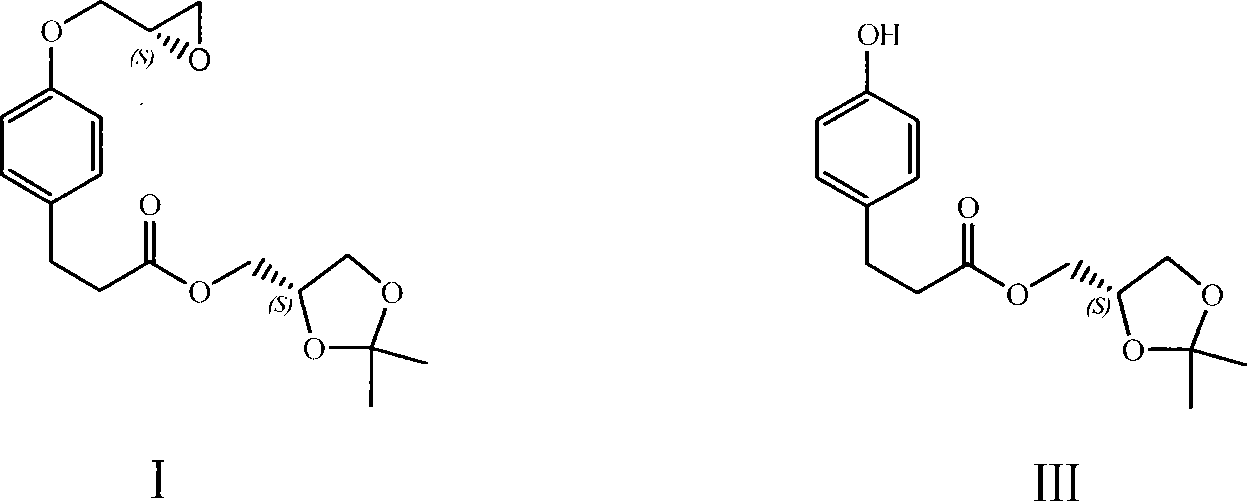
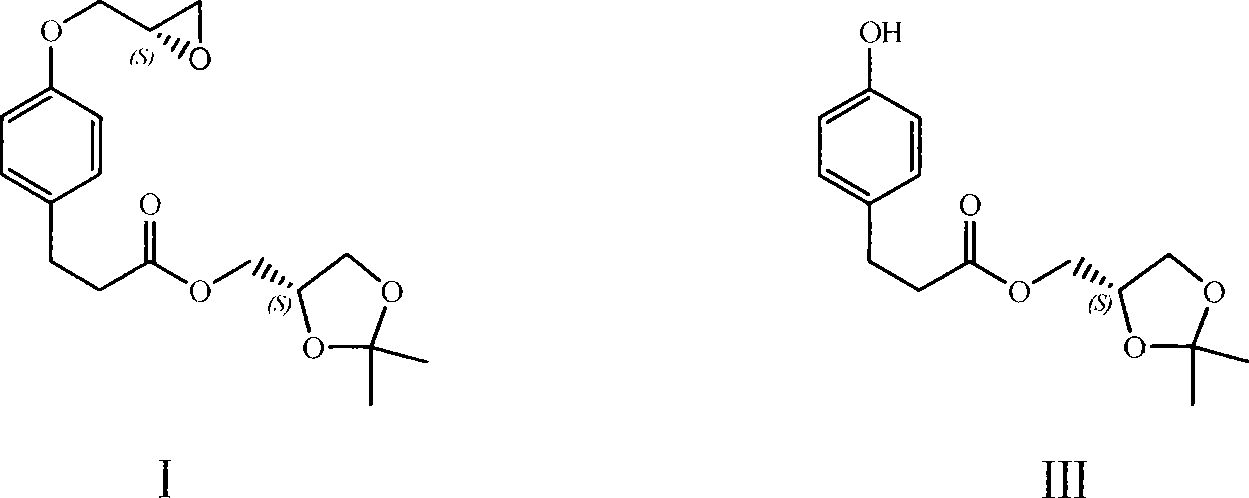
[0015] Add 5g (2,2-dimethyl-1,3-dioxo-4S-heterocyclic)methyl-3-[4-hydroxyphenyl]propionate (compound of formula III) to a 100ml three-necked flask, 6.2 g of anhydrous potassium carbonate and 60ml of acetonitrile, stirred to dissolve. Heat to reflux. Under reflux conditions, 5g (R)-epichlorohydrin was added dropwise. After dropping, the mixture was refluxed for 14 hours, and the reaction was detected by TLC. After the reaction was completed, suction filtered, and the filtrate was concentrated under reduced pressure to obtain a pink oil. The oil was dissolved in ethyl acetate, washed with saturated brine, and dried over anhydrous sodium sulfate for 1 hour. Suction filtration, the filtrate was concentrated under reduced pressure to obtain 4.9g oily matter, i.e. (2,2-dimethyl-1,3-dioxo-4S-heterocyclic)methyl-3-[4-(2(S), 3-epoxypropyl)phenyl]propionate (compound of formula I), the yield is 90%. The purity is 97.5% (HPLC).
reference example 1
[0016] Reference Example 1 (Chem.Pharm.Bull.40(6)1462-1469(1992))
[0017] Add 1.23g (2,2-dimethyl-1,3-dioxo-4S-heterocycle)methyl-3-[4-hydroxyphenyl]propionate, 1.00g (2S) -(+)-Glycidyl tosylate, 1.21g of anhydrous potassium carbonate and 10ml of anhydrous N,N-dimethylformamide were stirred to dissolve. Heat to 70°C, and continue heating at 70°C for 15 hours. After the reaction was completed, 60 ml of ethyl acetate was added to the reaction solution, washed with distilled water and brine in turn, and the organic phase was dried over anhydrous magnesium sulfate. Suction filtration, the filtrate was concentrated under reduced pressure, and the oil was obtained for column chromatography (eluent: dichloromethane-ethyl acetate volume ratio 9:1) to obtain 1.18g of the target compound, namely (2,2-dimethyl- 1,3-Dioxy-4S-heterocycle)methyl-3-[4-(2(S),3-epoxypropyl)phenyl]propionate, the yield was 80%.
reference example 2
[0018] Reference example 2 (EP 0397031)
[0019] Add 950mg (2,2-dimethyl-1,3-dioxo-4S-heterocycle)methyl-3-[4-hydroxyphenyl]propionate, 0.58ml(R)- Epibromohydrin, 1.406g of anhydrous potassium carbonate and 15ml of acetone were stirred to dissolve. Heat to reflux. Reflux for 16 hours. TLC detection reaction. After the reaction was completed, suction filtered, and the filtrate was concentrated under reduced pressure to obtain an oil. The oil was dissolved in water and the organic phase was extracted with ethyl acetate. The organic phase was washed successively with distilled water and saturated brine, and dried over anhydrous magnesium sulfate. Suction filtration, the filtrate was concentrated under reduced pressure, and the oil was obtained for column chromatography (eluent: ethyl acetate-dichloromethane volume ratio 4:96), to obtain 869 mg of the target compound, namely (2,2-dimethyl-1 , 3-Dioxy-4S-heterocyclic)methyl-3-[4-(2(S),3-epoxypropyl)phenyl]propionate, the yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com